Abstract
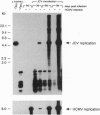
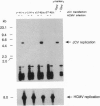
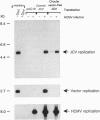
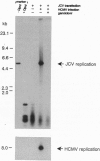
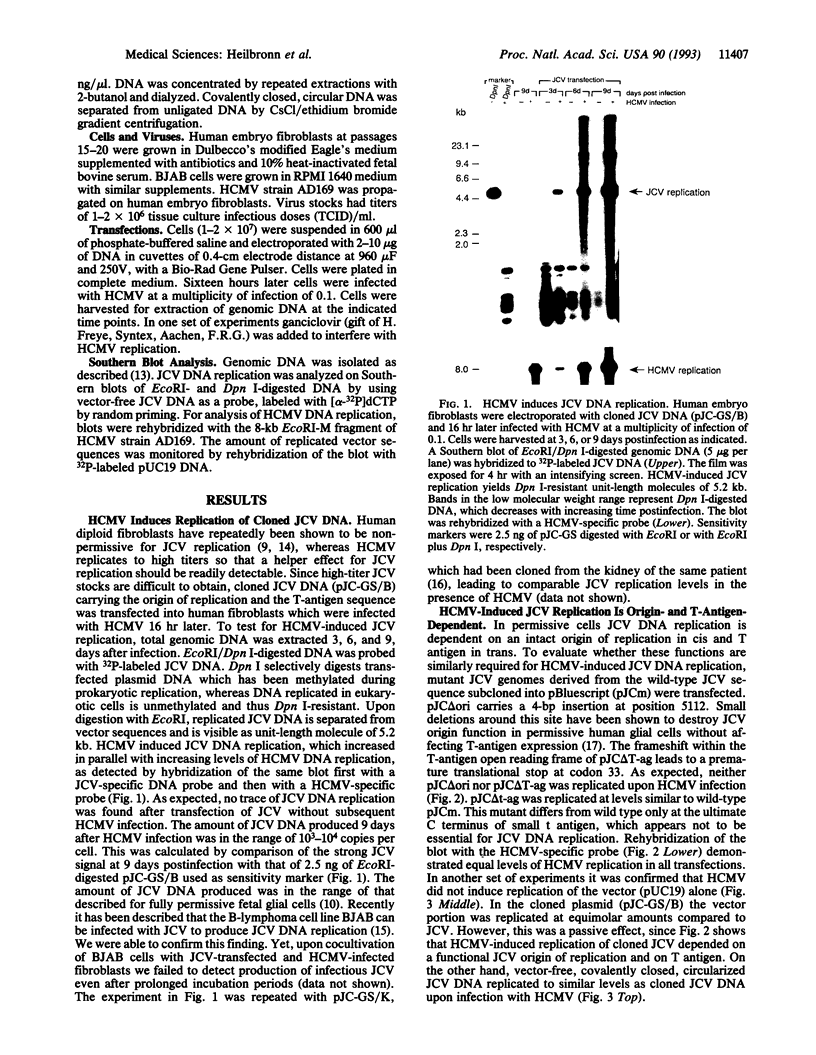
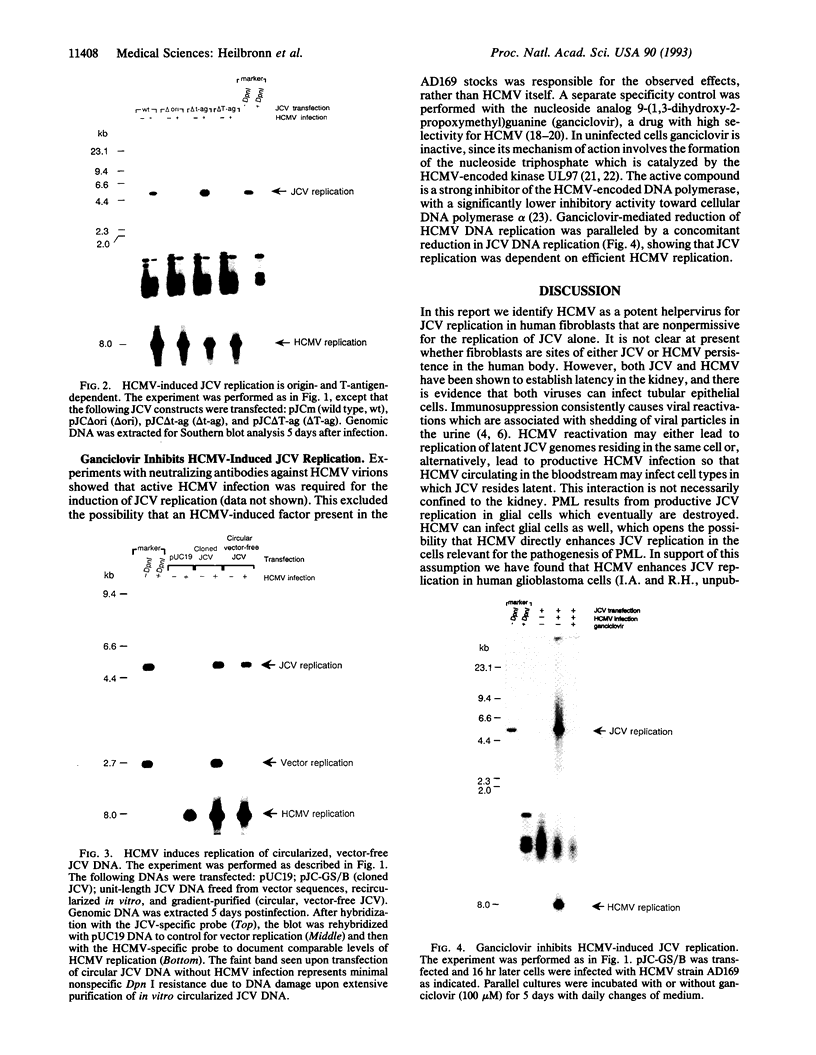
JC virus, a human papovavirus, is the causative agent of the demyelinating brain disease progressive multifocal leucoencephalopathy (PML). PML is a rare but fatal disease which develops as a complication of severe immunosuppression. Latent JC virus is harbored by many asymptomatic carriers and is transiently reactivated from the latent state upon immunosuppression. JC virus has a very restricted host range, with human glial cells being the only tissue in which it can replicate at reasonable efficiency. Evidence that latent human cytomegalovirus is harbored in the kidney similar to latent JC virus led to the speculation that during episodes of impaired immunocompetence, cytomegalovirus might serve as helper virus for JC virus replication in otherwise nonpermissive cells. We show here that cytomegalovirus infection indeed leads to considerable JC virus DNA replication in cultured human fibroblasts that are nonpermissive for the replication of JC virus alone. Cytomegalovirus-mediated JC virus replication is dependent on the JC virus origin of replication and T antigen. Ganciclovir-induced inhibition of cytomegalovirus replication is associated with a concomitant inhibition of JC virus replication. These results suggest that reactivation of cytomegalovirus during episodes of immunosuppression might lead to activation of latent JC virus, which would enhance the probability of subsequent PML development. Ganciclovir-induced repression of both cytomegalovirus and JC virus replication may form the rational basis for the development of an approach toward treatment or prevention of PML.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AbuBakar S., Au W. W., Legator M. S., Albrecht T. Induction of chromosome aberrations and mitotic arrest by cytomegalovirus in human cells. Environ Mol Mutagen. 1988;12(4):409–420. doi: 10.1002/em.2860120409. [DOI] [PubMed] [Google Scholar]
- Albrecht T., Nachtigal M., St Jeor S. C., Rapp F. Induction of cellular DNA synthesis and increased mitotic activity in syrian hamster embryo cells abortively infected with human cytomegalovirus. J Gen Virol. 1976 Feb;30(2):167–177. doi: 10.1099/0022-1317-30-2-167. [DOI] [PubMed] [Google Scholar]
- Anders D. G., Irmiere A., Gibson W. Identification and characterization of a major early cytomegalovirus DNA-binding protein. J Virol. 1986 May;58(2):253–262. doi: 10.1128/jvi.58.2.253-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders D. G., Kacica M. A., Pari G., Punturieri S. M. Boundaries and structure of human cytomegalovirus oriLyt, a complex origin for lytic-phase DNA replication. J Virol. 1992 Jun;66(6):3373–3384. doi: 10.1128/jvi.66.6.3373-3384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders D. G., Kidd J. R., Gibson W. Immunological characterization of an early cytomegalovirus single-strand DNA-binding protein with similarities to the HSV major DNA-binding protein. Virology. 1987 Dec;161(2):579–588. doi: 10.1016/0042-6822(87)90154-1. [DOI] [PubMed] [Google Scholar]
- Anders D. G., Punturieri S. M. Multicomponent origin of cytomegalovirus lytic-phase DNA replication. J Virol. 1991 Feb;65(2):931–937. doi: 10.1128/jvi.65.2.931-937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood W. J., Amemiya K., Traub R., Harms J., Major E. O. Interaction of the human polyomavirus, JCV, with human B-lymphocytes. Virology. 1992 Oct;190(2):716–723. doi: 10.1016/0042-6822(92)90909-9. [DOI] [PubMed] [Google Scholar]
- Biron K. K., Fyfe J. A., Stanat S. C., Leslie L. K., Sorrell J. B., Lambe C. U., Coen D. M. A human cytomegalovirus mutant resistant to the nucleoside analog 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine (BW B759U) induces reduced levels of BW B759U triphosphate. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8769–8773. doi: 10.1073/pnas.83.22.8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron K. K., Stanat S. C., Sorrell J. B., Fyfe J. A., Keller P. M., Lambe C. U., Nelson D. J. Metabolic activation of the nucleoside analog 9-[( 2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovich R. M., Frenkel N. Herpes simplex virus induces the replication of foreign DNA. Mol Cell Biol. 1988 Aug;8(8):3272–3281. doi: 10.1128/mcb.8.8.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörries K. Progressive multifocal leucoencephalopathy: analysis of JC virus DNA from brain and kidney tissue. Virus Res. 1984 Jan;1(1):25–38. doi: 10.1016/0168-1702(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Elsner C., Dörries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992 Nov;191(1):72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- Ertl P. F., Powell K. L. Physical and functional interaction of human cytomegalovirus DNA polymerase and its accessory protein (ICP36) expressed in insect cells. J Virol. 1992 Jul;66(7):4126–4133. doi: 10.1128/jvi.66.7.4126-4133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas V. R., Smee D. F., Chernow M., Boehme R., Matthews T. R. Activity of 9-(1,3-dihydroxy-2-propoxymethyl)guanine compared with that of acyclovir against human, monkey, and rodent cytomegaloviruses. Antimicrob Agents Chemother. 1985 Aug;28(2):240–245. doi: 10.1128/aac.28.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisque R. J., Martin J. D., Padgett B. L., Walker D. L. Infectivity of the DNA from four isolates of JC virus. J Virol. 1979 Nov;32(2):476–482. doi: 10.1128/jvi.32.2.476-482.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzeh F. M., Lietman P. S., Gibson W., Hayward G. S. Identification of the lytic origin of DNA replication in human cytomegalovirus by a novel approach utilizing ganciclovir-induced chain termination. J Virol. 1990 Dec;64(12):6184–6195. doi: 10.1128/jvi.64.12.6184-6195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn R., zur Hausen H. A subset of herpes simplex virus replication genes induces DNA amplification within the host cell genome. J Virol. 1989 Sep;63(9):3683–3692. doi: 10.1128/jvi.63.9.3683-3692.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. III. Virus-induced DNA polymerase. J Virol. 1975 Aug;16(2):298–310. doi: 10.1128/jvi.16.2.298-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T., Kitamura T., Guo J., Taguchi F., Aso Y., Nagashima K., Yogo Y. Origin of JC polyomavirus variants associated with progressive multifocal leukoencephalopathy. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5062–5065. doi: 10.1073/pnas.90.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble G. W., McCormick A. L., Pereira L., Mocarski E. S. A cytomegalovirus protein with properties of herpes simplex virus ICP8: partial purification of the polypeptide and map position of the gene. J Virol. 1987 Oct;61(10):3143–3151. doi: 10.1128/jvi.61.10.3143-3151.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler E., Stuart A. D., Chee M. S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992 Jul 9;358(6382):160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- Loeber G., Dörries K. DNA rearrangements in organ-specific variants of polyomavirus JC strain GS. J Virol. 1988 May;62(5):1730–1735. doi: 10.1128/jvi.62.5.1730-1735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K. J., Frisque R. J. Identification of critical elements within the JC virus DNA replication origin. J Virol. 1990 Dec;64(12):5812–5822. doi: 10.1128/jvi.64.12.5812-5822.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major E. O., Miller A. E., Mourrain P., Traub R. G., de Widt E., Sever J. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1257–1261. doi: 10.1073/pnas.82.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl C., Walker D. L., Frisque R. J. Derivation and characterization of POJ cells, transformed human fetal glial cells that retain their permissivity for JC virus. J Virol. 1987 Mar;61(3):755–763. doi: 10.1128/jvi.61.3.755-763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Chiou J. F., Cheng Y. C., Huang E. S. Human cytomegalovirus-induced DNA polymerase and its interaction with the triphosphates of 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)-5-methyluracil, -5-iodocytosine, and -5-methylcytosine. J Virol. 1985 Dec;56(3):846–851. doi: 10.1128/jvi.56.3.846-851.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Chiou J. F., Cheng Y. C., Huang E. S. Inhibition of cellular DNA polymerase alpha and human cytomegalovirus-induced DNA polymerase by the triphosphates of 9-(2-hydroxyethoxymethyl)guanine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J Virol. 1985 Mar;53(3):776–780. doi: 10.1128/jvi.53.3.776-780.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz B. Herpes simplex virus causes amplification of recombinant plasmids containing simian virus 40 sequences. J Gen Virol. 1989 Jun;70(Pt 6):1347–1358. doi: 10.1099/0022-1317-70-6-1347. [DOI] [PubMed] [Google Scholar]
- Pari G. S., St Jeor S. C. Effect of human cytomegalovirus on replication of SV40 origin and the expression of T antigen. Virology. 1990 Aug;177(2):824–828. doi: 10.1016/0042-6822(90)90558-9. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Hausen J. Z. Induction of mutations within the host cell genome by partially inactivated herpes simplex virus type 1. Virology. 1982 Oct 30;122(2):471–475. doi: 10.1016/0042-6822(82)90247-1. [DOI] [PubMed] [Google Scholar]
- Stoner G. L., Walker D. L., Webster H. D. Age distribution of progressive multifocal leukoencephalopathy. Acta Neurol Scand. 1988 Oct;78(4):307–312. doi: 10.1111/j.1600-0404.1988.tb03661.x. [DOI] [PubMed] [Google Scholar]
- Sullivan V., Talarico C. L., Stanat S. C., Davis M., Coen D. M., Biron K. K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992 Jul 9;358(6382):162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- Tada H., Rappaport J., Lashgari M., Amini S., Wong-Staal F., Khalili K. Trans-activation of the JC virus late promoter by the tat protein of type 1 human immunodeficiency virus in glial cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3479–3483. doi: 10.1073/pnas.87.9.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Challberg M. D., Nelson N. J., Levine M., Glorioso J. C. Inversion events in the HSV-1 genome are directly mediated by the viral DNA replication machinery and lack sequence specificity. Cell. 1988 Jul 29;54(3):369–381. doi: 10.1016/0092-8674(88)90200-0. [DOI] [PubMed] [Google Scholar]
- White F. A., 3rd, Ishaq M., Stoner G. L., Frisque R. J. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992 Oct;66(10):5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Kitamura T., Sugimoto C., Hara K., Iida T., Taguchi F., Tajima A., Kawabe K., Aso Y. Sequence rearrangement in JC virus DNAs molecularly cloned from immunosuppressed renal transplant patients. J Virol. 1991 May;65(5):2422–2428. doi: 10.1128/jvi.65.5.2422-2428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Kitamura T., Sugimoto C., Ueki T., Aso Y., Hara K., Taguchi F. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. J Virol. 1990 Jun;64(6):3139–3143. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]