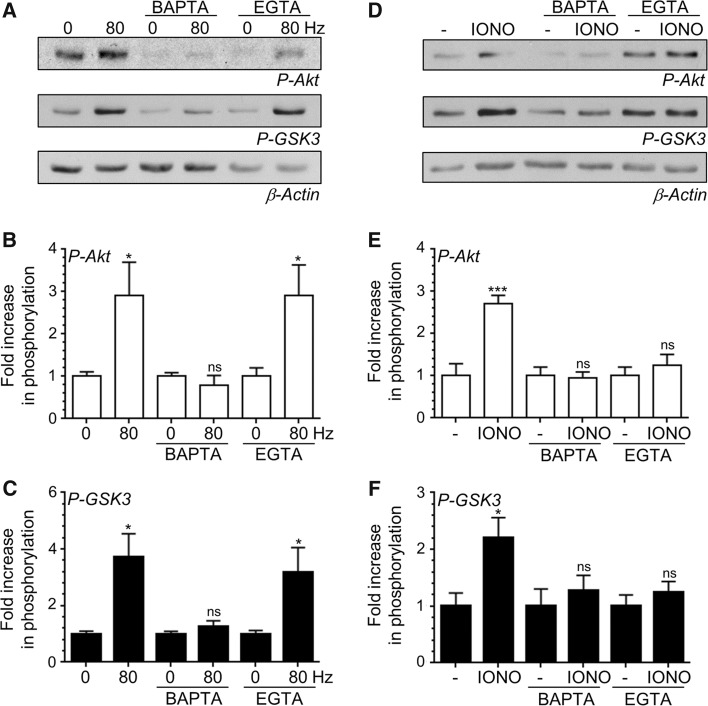
Fig. 3.
Localised Ca2+ influx is essential for Akt/GSK3 phosphorylation. CGNs were removed from culture medium and repolarised in incubation medium for 10 min. Cultures were then incubated with or without incubation medium supplemented with either 100 μM BAPTA-AM or EGTA-AM for 30 min. After this point CGNs were left to rest or challenged with either 800 action potentials (80 Hz) or ionomycin (IONO, 2.5 µM for 1 min). a, d Representative blots are displayed showing either Akt Ser473 phosphorylation (P-Akt), GSK3α/β Ser21/9 phosphorylation (P-GSK3) or β-Actin levels (β-Actin) after either action potential (a) or ionomycin (d) stimulation. b, e The fold increase in phosphorylation of Akt Ser473 (open bars) in response to either action potentials (b) or ionomycin (e) is displayed. c, f The fold increase in phosphorylation of GSK3α/β Ser21/9 (closed bars) in response to either action potentials (c) or ionomycin (f) is displayed. In all cases phosphorylation levels were corrected for protein levels using β-Actin and normalisation to the basal controls. All error bars represent ±SEM; n = 4 for P-Akt 80 Hz, n = 5 for P-GSK3 80 Hz, n = 7 for P-Akt IONO and n = 6 for P-GSK3 IONO (students t test, ns non-significant *p < 0.05; **p < 0.01, ***p < 0.001 basal to 80 Hz or basal to ionomycin per condition)