Although adenomyosis is a largely benign disease, malignant transformations do happen in rare cases, resulting in endometrioid adenocarcinoma or sarcoma in the myometrium. Recently, attention has been focused on the malignant transformation of the endometriosis. In 1988, the concept of atypical endometriosis (aEM) was first proposed by LaGrenade and Silverberg,[1] who suggested that aEM might be a precursor of malignant transformations of endometriosis. However, the majority of investigations of aEM focus on endometriosis, while adenomyosis remains understudied. In this article, we report 10 cases of atypical glandular hyperplasia transformation of adenomyosis, of whom the clinical and pathological characteristics are observed.
PATIENTS
From January 2007 to December 2014, among the 1077 patients with adenomyosis at People's Hospital, Peking University, China, 10 patients (0.93%) were diagnosed with atypical glandular hyperplasia transformation of adenomyosis. The mean age of these patients was 43 years (39–56 years). Eight patients (8/10) were premenopausal and 2 (2/10) were postmenopausal. Two gynecological and pathological specialists reviewed sections of pathological tissue and confirmed the initial diagnosis.
DIAGNOSIS
Eight patients were found after surgery to have endometrial diseases. Six were diagnosed as endometrioid carcinoma of the following FIGO stages: 3 cases of IaG1, 2 cases of IaG2, and 1 case of IbG1. Among them, two had no myometrial invasion, four had superficial myometrial invasion (<1/2 myometrial invasion). Four patients received pelvic and paraaortic lymphadenctomy and none had lymph node metastasis. The other 2 patients who were not undertaken lymphadenctomy had pelvic MRI after the operation, and neither of the results indicated pelvic nor paraaortic lymph nodes metastasis. Two cases were diagnosed as atypical endometrial hyperplasia.
CLINICAL SYMPTOMS AND MEDICAL HISTORIES
The common clinical symptoms included abnormal uterine bleeding (8 cases) and pelvic mass (3 cases). Four patients experienced irregular menstruation while 4 had regular periods, and 7 patients had dysmenorrhea. The mean number of pregnancies and mean delivery times were 2 (0–4) and 1 (0–2) time, respectively. Two patients experienced primary infertility while 2 patients experienced secondary infertility, and 1 patient had a history of hydatidiform moles. Four patients had primary hypertension, 2 patients had diabetes mellitus, 1 patient had fatty liver disease, and 4 patients had anemia. There was no family history of uterine, breast, or ovarian cancer in this cohort of 10 patients, and no patients reported taking birth control pills or estrogen replacement therapy.
ACCESSORY EXAMINATIONS
Nine patients had detectable serum CA-125 levels before surgery with a mean value of 104.50 U/L (range, 11.38–357.40 U/L); 6 cases presented a value above 35.00 U/L. Transvaginal ultrasound images before operation showed adenomyosis in 9 patients, and magnetic resonance imaging suggested adenomyosis in 1 patient; neither method suggested any abnormal signal at the focus. Dilatation and curettage under hysteroscopy were performed in 6 patients before surgery, and pathology revealed 2 cases of endometrioid carcinoma, 3 cases of endometrial atypical hyperplasia not exclusive of carcinoma, and 1 case of complex hyperplasia. All the 10 patients were found to have adenomyosis by pathological examination after surgery, and atypical glandular epithelial hyperplasia was found at the adenomyosis focus.
OPERATIVE TREATMENTS
All patients underwent surgery: 9 patients received a total hysterectomy and bilateral salpingo-oophorectomy, 1 patient received a total hysterectomy alone, and 4 patients received a pelvic lymphadenectomy. Five patients received laparoscopic surgery while 5 patients received laparotomy. After surgery, 4 patients were administered oral progesterone for 6–12 months due to diffuse foci.
PATHOLOGICAL CHARACTERISTICS
The pathological characteristics of atypical glandular hyperplasia transformation of adenomyosis included such features as increased endometrial gland size in the focus of the adenomyosis with an irregular outline and decreased mesenchyme; and mild to moderate atypical glandular epithelial hyperplasia, which presented as disordered or undetectable cellular polarity, nuclear enlargement, positional reversal, irregular cellular shape, and a visible nucleolus. All these characteristics were similar to those of atypical hyperplasia of the endometrium in situ. However, we observed neither glandular fusion nor severe abnormalities in the focus, and the endometrial mesenchyme was still present and had not infiltrated the surrounding smooth muscle cells with atypical glandular cells, which were the primary distinctions from carcinoma [Figure 1].
Figure 1.
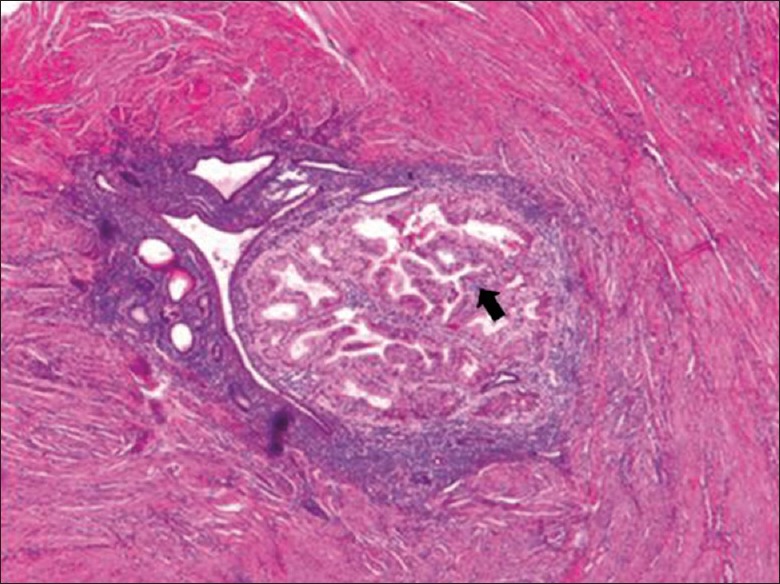
Adenomyosis, in some area, the glands were arranged tightly with different sizes and the cytoplasm was eosinophilic, that presented a picture of atypical hyperplasia (arrow) (H and E, original magnification ×40).
FOLLOW-UP
All the patients were subjected to a follow-up interview in the clinic or by telephone. The median follow-up time was 41 months (17–90 months), and the follow-up rate was 100%. All patients were alive and without relapse as of March 2015.
DISCUSSION
In 1925, Sampson[2] was the first to document cases of suspected malignant transformation of endometriosis. The overall frequency of malignant transformation of endometriosis is estimated to be between 0.7% and 1.0%, with a relative risk ranging between 1.3% and 1.9%.[3] By contrast, the malignant transformation of adenomyosis is rare, and the phenomenon has only rarely been described in case reports.
In 1988, LaGrenada and Silverberg[1] published a case study of five ovarian tumors associated with aEM in which they proposed the concept of aEM. The rate of aEM occurrence has been reported to be 1.7–3.0% by different studies. Given the evidence that endometriosis is a common gynecologic disorder occurring in approximately 7–10% of women of reproductive age, aEM should be the subject of greater attention.
However, reports detailing atypical hyperplasia in ectopic endometrium primarily discuss ovarian endometriosis and seldom focus on endometriosis of extraovarian location. It has been reported that 54–90% patients with adenomyosis are likely to exhibit pelvic endometriosis, which suggests that the two diseases are indeed closely related.[4] In this article, atypical glandular hyperplasia transformation of adenomyosis was reported to occur in 0.93% patients with adenomyosis. Whether this condition should be classified as aEM or another disorder remains to be determined by gynecologists and pathologists. For the sake of discussion, atypical glandular hyperplasia is categorized as aEM in this article.
The diagnosis of aEM is based on the histopathological criteria recommended by LaGrenade and Silverberg.[1] These features include eosinophilic cytoplasm; large hyperchromatic or pale nuclei with moderate to marked pleomorphism; an increased nuclear to cytoplasmic ratio; and cellular crowding and stratification or tufting. Cases that meet three or more of these criteria are classified as aEM.
Atypical hyperplasia was identified by these criteria in the foci of the 10 adenomyosis cases presented in this report. Four patients with endometrial adenocarcinoma exhibited superficial muscular infiltration, but the morphology of the endometrial adenocaricoma focus was different from that of atypical glandular hyperplasia of adenomyosis, and normal smooth-muscle cells existed between two different lesions. In addition, glandular structures were maintained in the atypical hyperplasia glands of adenomyosis without fusion or infiltration to the surrounded smooth-muscle cells.
The pathogenesis of aEM is not yet characterized, current consensus suggests that aEM might be a precursor of some ovarian cancers and could progress to malignancy. Research detailing the mechanisms of malignant transformation is mostly focused on tumor suppressor gene inactivation; loss of heterozygosity; excess accumulation of heme and free iron in endometriotic lesions; increased levels of cytokines and growth factors; high levels of aromatase activity; unbalanced steroid hormone levels; and systematic or topical immune abnormalities.[5]
Malignant transformations of adenomyosis are extremely rare, and their pathogenesis is still under investigation. A number of hormonal, genetic, immunological, and growth factors may play a role.
In conclusion, atypical glandular hyperplasia transformation of adenomyosis is rarely observed in clinics. Because it may be closely related to endometrial disease in situ and to malignant transformations of adenomyosis, it is worthy of more attention from gynecologists and pathologists. To propose a standard diagnosis and treatment plan, it is necessary to investigate the clinicopathological characteristics of adenomyosis further.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Hum Pathol. 1988;19:1080–4. doi: 10.1016/s0046-8177(88)80090-x. doi: 10.1016/S0046-8177(88)80090-X. [DOI] [PubMed] [Google Scholar]
- 2.Sampson J. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg. 1925;10:1–72. [Google Scholar]
- 3.Lang J. Research and consideration of endometriosis (in Chinese) Chin J Obstet Gynecol. 2003;38:478–80. [Google Scholar]
- 4.Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecol Oncol. 2012;124:164–9. doi: 10.1016/j.ygyno.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Prefumo F, Todeschini F, Fulcheri E, Venturini PL. Epithelial abnormalities in cystic ovarian endometriosis. Gynecol Oncol. 2002;84:280–4. doi: 10.1006/gyno.2001.6529. doi: 10.1006/gyno.2001.6529. [DOI] [PubMed] [Google Scholar]


