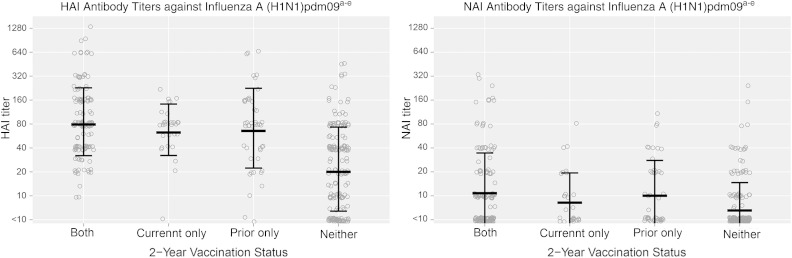
Figure 1.
Distributions of pre-season susceptibility titers of hemagglutination-inhibition (HAI) and neuraminidase-inhibition (NAI) antibody against 2009 pandemic influenza A(H1N1) virus, based on each combination of current-season and prior-season vaccine exposure. aAntibody titers measured by hemagglutination-inhibition (HAI) and neuraminidase-inhibition (NAI) assays in sera collected from a subset of subjects aged ≥13 years at pre-season visits (or at enrollment for those subjects without pre-season specimens and no evidence of influenza vaccine receipt) were used to estimate pre-season susceptibility to influenza; bSera were tested with the HAI assay using as the antigen the influenza A (pH1N1)pdm09 virus strain present in the 2013–2014 North American influenza vaccine (A/California/07/2009); cSera were tested with the NAI assay using as the target, a reassortant influenza virus with the NA representing the A (pH1N1)pdm09 virus strain present in the 2013–2014 North American influenza vaccine (A/California/07/2009) and a mismatched HA (H6 subtype); dEach circle indicates the titer of an individual observation; Linked lines indicate the geometric mean titer ± the geometric standard deviation; eAll vaccination groups (both years, current only, and prior only) had significantly higher geometric mean titers (P < .001) than those unvaccinated both years for all antigens.