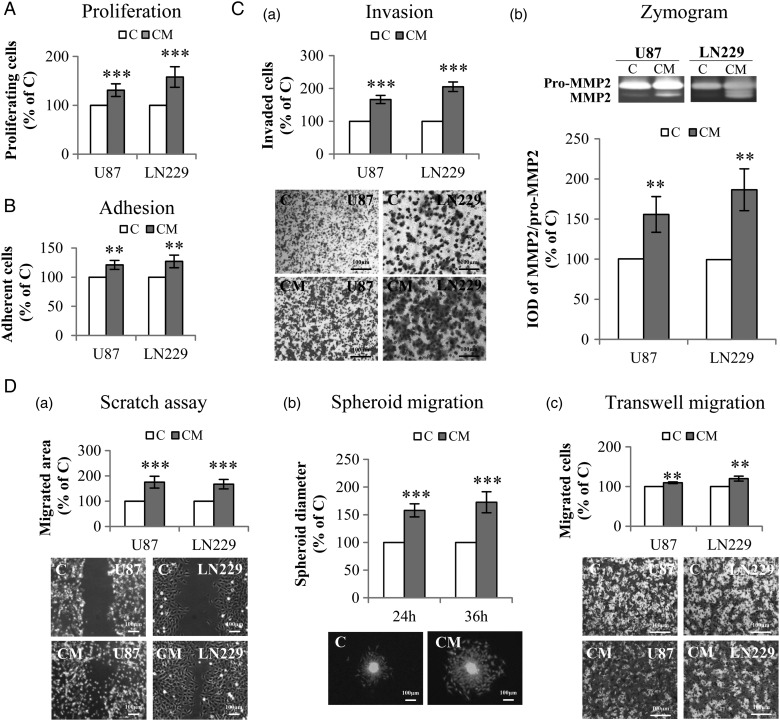
Fig. 2.
Indirect co-culture of glioblastoma (GBM) cells with PDCD10-silenced human umbilical vein endothelial cells (HUVECs) stimulated GBM cell proliferation, adhesion, invasion, and migration. Indirect co-culture was achieved by the culture of GBM cells with conditioned medium (CM) or with control medium (C), collected from HUVECs at 72 hours after the transfection with siPDCD10 and control siRNA respectively. (A) GBM cell proliferation detected by WST-1 assay. (B) GBM cell adhesion. Cell adhesion was determined by crystal-violet staining followed by measuring the absorbance at 550 nm. (C) CM facilitated invasion of GBM cells (a) accompanied with activation of MMP2 (b). U87 or LN229 were treated with CM or control medium for 24 hours. Prestimulated cells were then seeded in 24-well transwell plates coated with Matrigel. Cell invasion was quantified 24 hours after the incubation by crystal-violet staining (a); meanwhile, the culture medium of prestimulated cells was collected for gelatin zymography (b). (D) GBM cell mobility was measured by scratch assay (a), spheroid migration assay (b), and transwell migration assay (c). For scratch assay (a), cell migration was recorded 10 hours after scratching. To perform spheroid migration assay (b), the spheroids formed by LN:GFP were suspended in CM or in control medium, and seeded on 96-well plates precoated with Matrigel. For transwell migration assay (c), U87 or LN229 were suspended in serum-free medium and added into the insert of 24-well transwell plates. CM or control medium was added into the lower chamber. After incubation for 24 hours, the migrated cells were detected after crystal-violet staining. IOD: integrated optical density. ** P < .01 and *** P < .001, compare to C. Scale bar: 100 µm.