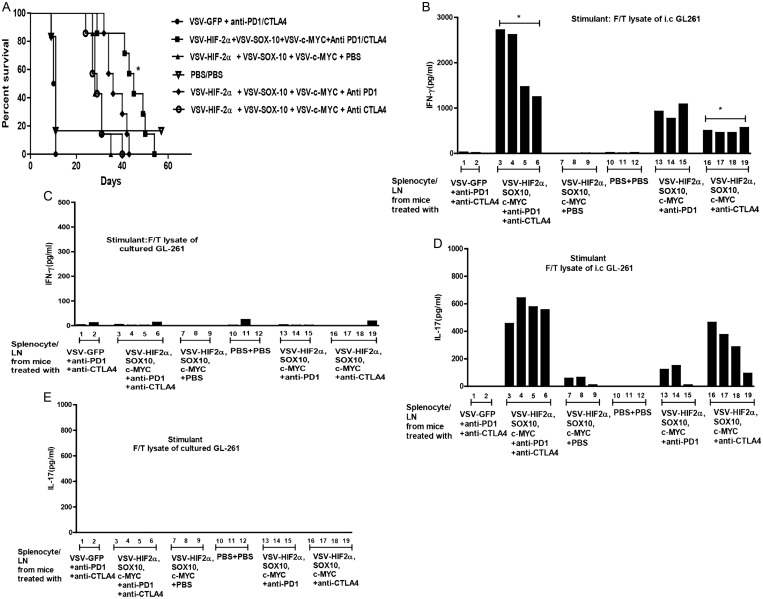
Fig. 6.
Double checkpoint inhibition therapy enhances treatment with VSV-TAA. (A) C57BL/6 mice bearing 5-day established i.c. GL261 tumors were treated intravenously (n = 7–8 mice per group) with a total dose of 5 × 106 pfu of VSV-GFP; VSV-HIF-2α, VSV-Sox-10, and VSV-c-Myc, or PBS on days 6, 8, and 10 and 13, 15, and 17, respectively. On days 13, 15, and 17 these groups were also treated with either anti-PD1 antibody, anti-CTLA4 antibody, anti-PD1 antibody + anti-CTLA4 antibody, or PBS as shown. Survival with time is shown. Representative of 2 separate experiments. *P = .0015 between VSV-HIF-2α, VSV-Sox-10, and VSV-c-Myc and VSV–HIF-2α, VSV–Sox-10, and VSV–c-Myc + anti-PD1 and anti-CTLA4. (B–E) Splenocytes and lymph nodes were pooled from 3 C57BL/6 mice per group bearing 5-day established i.c. GL261 tumors treated with either VSV-GFP + anti-PD1 + anti-CTLA4; VSV–HIF-2α, VSV–Sox-10, and VSV–c-Myc + anti-PD1 antibody + anti-CTLA4 antibody; VSV–HIF-2α, VSV–Sox-10, and VSV–c-Myc + PBS; PBS + PBS; VSV–HIF-2α, VSV–Sox-10, and VSV–c-Myc + anti-PD1 antibody; or VSV–HIF-2α, VSV–Sox-10, and VSV–c-Myc + anti-CTLA4 antibody. Cells were plated at 1 × 106 cells per well and restimulated in vitro 3 times at 24-h intervals with 1 × 105 cells of freeze/thaw lysates of GL261 tumors recovered from mice bearing i.c. GL261 tumors (B and D) or with freeze/thaw lysates of in vitro cultured GL261 (C and E). Forty-eight hours later, supernatants were assayed for IFN-γ (B and C) or IL-17 (D and E) by ELISA. (B) *P = .0282 comparing lanes 3–6 and 16–19.