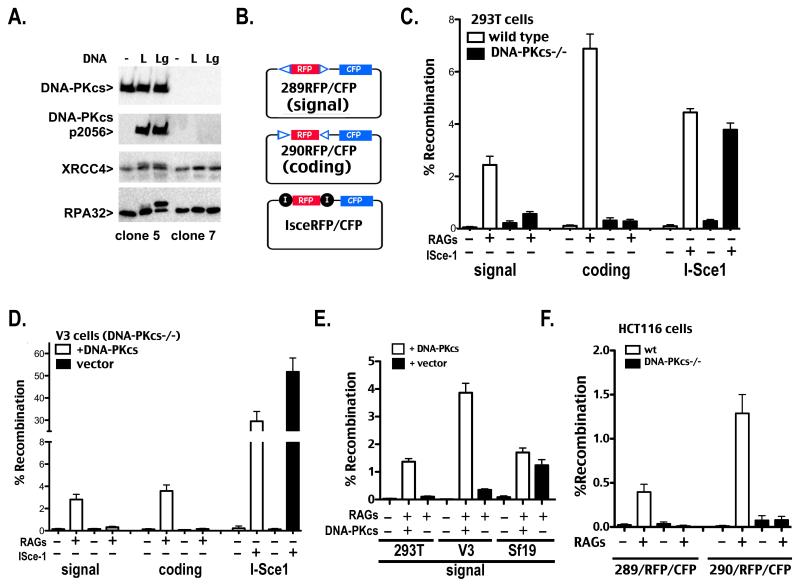
Figure 1. Human 293T cells that lack DNA-PKcs are severely deficient in signal end joining.
A. In vitro kinase assays were performed as described in Methods. Briefly, DNA-PK was not activated (−), activated with linear DNA (L), or activated with linear DNA that includes a single stranded gap (Lg). Kinase reactions were analyzed by western blotting using antibodies specific for DNA-PKcs, phospho-2056 DNA-PKcs, XRCC4, or RPA32. B. Schematic of plasmid VDJ recombination substrates used to detect signal joints (top), coding joints (middle), or I-Sce1 DSB-induced deletion (bottom) are depicted. C and D. Wild type and DNA-PKcs-deficient 293T (C) or V3 (D) cells were transfected with the indicated recombination substrate as well as RAG1 and RAG2 or an I-Sce1 expression construct as specified. Cells were analyzed by flow cytometry 72 hr after transfection and the percentage of recombination was calculated as the percentage of live cells expressing CFP divided by the percentage expressing RFP. Error bars represent the standard error of the means. E. DNA-PKcs-deficient 293T, V3, or Sf19 cells were transfected with indicated recombination substrates, RAG1 and RAG2, and the DNA-PKcs expression construct as specified. Cells were analyzed by flow cytometry as in C and D. F. Wild type and DNA-PKcs-deficient HCT116 cells were transfected with the indicated recombination substrate as well as RAG1 and RAG2 expression constructs as specified. Cells were analyzed by flow cytometry 72 hr after transfection and the percentage of recombination was calculated as the percentage of live cells expressing CFP divided by the percentage expressing RFP. Error bars represent the standard error of the means.