Figure 4.
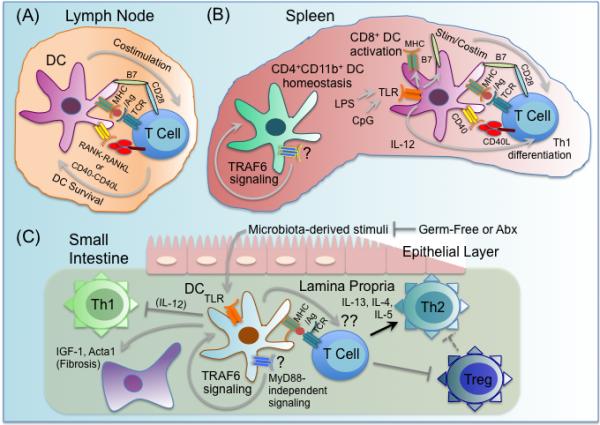
Multiple roles for TRAF6 in dendritic cell function. TRAF6 has multiple important roles in dendritic cell (DC) biology, including regulating DC activation, survival, homeostasis, and tolerance. (A) TRAF6 in DCs is a critical mediator of CD40 and RANK, which function to enhance DC survival and DC-T cell interactions in the lymph nodes. (B) TRAF6 signaling through an undetermined receptor(s) (?) is required for normal homeostasis of the splenic DC compartment, with TRAF6-deficient mice exhibiting a specific severe defect in the CD4+CD11b+ DC subset. Splenic DCs from TRAF6-deficient mice fail to upregulate MHC and costimulatory surface markers like B7-2/CD86, and fail to elaborate inflammatory cytokines like IL-12 in response to stimulation with either CD40L or various TLR ligands, including LPS and CpG DNA, likely due to TRAF6-dependent TLR and CD40 signaling. As a result, TRAF6-deficient DCs exhibit defective capacity to activate antigen-specific CD4 T cells to become IFNγ-producing Th1 cells. (C) TRAF6ΔDC mice, in which TRAF6 is specifically deleted in DCs, spontaneously develop MyD88-independent Th2-associated inflammation and fibrosis specifically in the small intestine, as well as diminished Treg numbers. Broad-spectrum antibiotics (Abx) treatment blocks development of TRAF6ΔDC intestinal phenotypes, while ablation of commensal microbiota via germ-free re-derivation exacerbates TRAF6ΔDC intestinal phenotypes. While microbiota-dependent TLR stimulation is likely TRAF6- and MyD88-dependent and required for lamina propria DC production of inflammatory cytokines like IL-12 that induce Th1 differentiation, TRAF6 signaling (but not MyD88 signaling) through an undetermined receptor(s), (?), is required for regulation of these DC-intrinsic processes. It is also unclear what DC TRAF6-dependent tolerigenic factor(s), (??), produced by lamina propria DCs that both promotes Tregs and inhibits spontaneous Th2 differentiation. It is possible that reduced Tregs leads to unrestrained Th2.
