Figure 1.
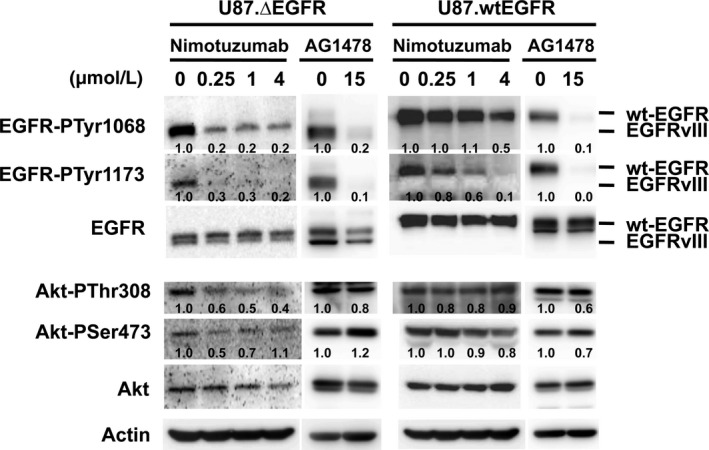
Dephosphorylation of EGFRvIII (∆EGFR) and wild‐type (wt) EGFR upon treatment with nimotuzumab or tyrphostin AG1478 in vitro. Cultured human glioma U87MG cells overexpressing either EGFRvIII (U87MG.∆EGFR) or wtEGFR (U87MG.wtEGFR) were treated with nimotuzumab for 72 h and their lysates were subjected to Western blot analysis. Nimotuzumab dephosphorylated EGFR at both tyrosine residues 1068 and 1173. EGFRvIII tyrosine phosphorylation was preferentially suppressed by nimotuzumab at lower doses, compared with wild‐type EGFR. Akt phosphorylation at threonine residue 308 was modestly suppressed in U87MG.∆EGFR cells by nimotuzumab. Relative tyrosine phosphorylation per molecule is shown below each lane, calculated as a ratio of that of untreated status and standardized with actin expression. A tyrphostin AG1478 was used as a positive control for EGFR inhibition.
