Abstract
Objective:
To measure length of hospital stay (LHS) in patients receiving medication reconciliation. Secondary characteristics included analysis of number of preadmission medications, medications prescribed at admission, number of discrepancies, and pharmacists interventions done and accepted by the attending physician.
Methods:
A 6 month, randomized, controlled trial conducted at a public teaching hospital in southern Brazil. Patients admitted to general wards were randomized to receive usual care or medication reconciliation, performed within the first 72 hours of hospital admission.
Results:
The randomization process assigned 68 patients to UC and 65 to MR. LHS was 10±15 days in usual care and 9±16 days in medication reconciliation (p=0.620). The total number of discrepancies was 327 in the medication reconciliation group, comprising 52.6% of unintentional discrepancies. Physicians accepted approximately 75.0% of the interventions.
Conclusion:
These results highlight weakness at patient transition care levels in a public teaching hospital. LHS, the primary outcome, should be further investigated in larger studies. Medication reconciliation was well accepted by physicians and it is a useful tool to find and correct discrepancies, minimizing the risk of adverse drug events and improving patient safety.
Keywords: Medication Reconciliation, Pharmaceutical Services, Randomized Controlled Trials as Topic, Brazil
INTRODUCTION
The transition between different care levels has been identified as one of the major critical points in hospital patient management. This is mainly attributed to deficiency of communication among healthcare professionals, patients, and their families1,2, and therefore represents a period of great vulnerability for patients’ safety.3 Vulnerability is attributed to three main factors: (a) numerous changes in pharmacotherapy during this period3; (b) low health literacy and/or low ability verbal communication3,4,5; and (c) poor transmission of information among physicians at different levels of care.3
One of the main causes of morbidity among patients admitted to the hospital is medication errors. Therefore, by the time of hospital admission, an accurate and thorough medication history collection should be conducted; otherwise, pharmacotherapy related problems, such as discrepancies, may occur, compromising treatment´s effectiveness and patient safety.6 Discrepancies are any differences between the medications taken by the patient prior to admission and medications prescribed at the hospital.7 A high prevalence of discrepancies was found at patient admission and approximately 67% of hospitalized patients experienced at least one error related to their past medication history.8 Recent efforts to improve the quality and safety of health care have included paying close attention to medication discrepancies that are encountered by patients who are receiving care across different settings.9
In order to minimize the risk of causing harm to patients, clinical pharmacists can manage the medication in collaboration with other professionals to optimize the pharmacotherapy.10 Clinical pharmacy services comprise discharge counseling, medication review, and medication reconciliation (MR), which is defined as “a formal process that consists of evaluating the complete and accurate list of medications previously used, along with the prescriptions after the transition, whose main objective is to eliminate possible discrepancies”.11 Countries such as Canada and the United States require the implementation of this service as a criterion for accreditation of health care institutions.12,13,14,15
In Brazil, discussion of MR and its implementation has become increasingly frequent. However, no clinical trial addressing this topic has been completed yet in our country, illustrating the need for more evidence to assess such services. The objective of this study was to evaluate the effects of introducing MR into the routine of the general wards in a southern Brazilian public teaching hospital.
METHODS
Design
This randomized, controlled trial included male and female patients older than 18 years newly admitted into general wards. Patients whose medication history was not collected within first the 72 hours of admission, patients who were discharged before completing 72 hours of admission, and patients who were transferred from the studied unit to another unit or hospital were excluded.
The study was conducted in a 6-month period, at Hospital de Clínicas da UFPR in southern Brazil. This public, university affiliated, teaching hospital has 370 beds and attends 3000 patients per day. A list of random numbers generated from Microsoft Excel was used for patient randomization. The random numbers were separated individually into MR and usual care group (UC) and correspond to the order of hospital admission.
To avoid bias the medication history was collected before the patient allocation in UC group by nursing staff and medical residents, and MR group by study clinical pharmacist. The patients were considered blind to the allocation, since all reconciliation interventions were targeted to attending physician without patient participation. The attending health professionals were not considered blind due to the interventions targeted to them.
This study was approved by the local research ethics committee and complies with the Helsinki Declaration.
Usual Care
Patients randomized to the UC received standard care provided at hospital admission. In this case the medication history was obtained, at least, by the nursing staff and medical residents, which excludes ethical implications. However, this process is not systematized, and does not have standardized registration method. For comparison with the MR, the UC was submitted only to step 1, described in Figure 1.
Figure 1.
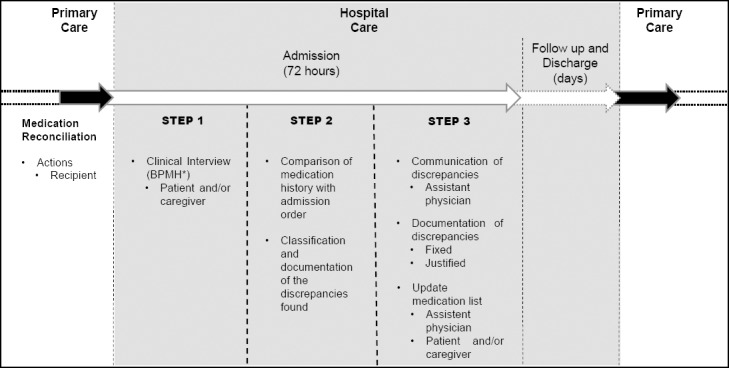
Description of Medication Reconciliation.4 * BPMH - Best Possible Medication History
Intervention
MR is schematically described in Figure 1 (step 1 to 3). A structured form was developed to record the data collected. Details were based on a novel tool, which describes pharmacist-led clinical services, entitled Descriptive Elements of Pharmacist Interventions Characterization Tool (DEPICT).16
During intervention, the recipients defined were the attending physician as health professional, and the patient and/or caregiver. With the patient and/or caregiver, the contact was made individually at bedside or in a room reserved for the multidisciplinary team. With attending physician, the contact was made in two ways: during the clinical rounds with the presence of other professionals of the team, or individually in a room reserved to physicians. The intervention was provided to all the patients enrolled and randomized to the MR, regardless of any health, social, or demographic condition.
During the procedure, access to the following sources of data was actively sought: drug prescription orders; pharmaceutical records or pharmacy computer system; medication list or brown bag data; patient self-monitoring data; adherence measuring tools; laboratory tests; patient interview; medical records; discharge or referral letter; contact with the health care professional; and clinical database. In order to standardize the data collection, pharmacists used a checklist developed for this study.
The parameters considered to design the intervention were the list of medications and the accuracy of the medication history, measured through the discrepancies found. When relevant, based on the discrepancies found, suggestions were given to the physician about changes in the pharmacotherapy and the patient’s list of medications was updated. The intervention was performed during patient admission. Contacts and actions took place within first 72 hours of admission.
A written action plan and the list of medications obtained through the data collection were used. Both were attached to the medical chart to support the suggestions made to the doctor. On average, two contacts were made during the entire procedure with each recipient: the patient and/or caregiver and the attending physician. However, the actions directed to the recipients were performed in a single day and in a single contact within first 72 hours of the admission period.
Communication during patient counseling was face-to-face without using any remote device. Communication with the attending physician was conducted primarily face-to-face and, in a few cases, remotely by written document attached to the medical chart. Suggestions for changes in pharmacotherapy were adjustment of medication dosage, interval, or route, and medication initiation. Changes in pharmacotherapy were made by the attending physician when the suggestions were accepted based on the discrepancies reported. In this action model, the pharmacist had no autonomy to make changes in the pharmacotherapy.
Data Collection
The variables collected to characterize the sample were: gender; age; number of comorbidities; distribution of comorbidities in clinical specialties; and Charlson Comorbidity Index. Descriptions of number of comorbidities and their classification in different specialties were used to qualitatively characterize the sample, indicating the complexity of individuals admitted into public hospitals. The Charlson Comorbidity Index is a tool that selects clinical conditions to calculate the risk of mortality.17
Primary outcome was length of hospital stay (LHS), which was collected through the hospital information system. This outcome was selected based on the Dooley´s study which results showed that MR is able to reduce LHS.18 Sample secondary characteristics were obtained through the collection and assessment of medication history, and included: (a) number of medications preadmission; (b) number of medications prescribed at admission; (c) number of discrepancies; and (d) number of interventions done and accepted. The characteristics (c) and (d) mentioned above were gathered only for the MR group.
Pharmacist interventions targeted the unintentional discrepancies. Discrepancies found in the MR were classified as intentional when the difference corresponded to an intentional change in the pharmacotherapy, and unintentional when the differences were not intentional and were considered medication errors.7 All unintentional discrepancies found in MR were classified as according to type: omission, dosage, interval, and route.
The interventions were conducted only on unintentional discrepancies in the MR that indicated a pattern regarding the type of discrepancy: (a) omission – start medication; (b) dosage – adjust dosage; (c) interval – adjust interval; and (d) route – adjust route. Interventions that resulted in correction within the period of first 72 hours of admission were considered accepted. Finally, to better characterize the service and its specificities, the following process variables were collected: available information sources to collect the medication history and time spent to perform the service.
Statistical Methods
Data consistency analysis was conducted to verify normality and presence of outliers. Whenever possible, non-normally distributed data were normalized. The following tests for parametric data were used: Student’s t test for continuous variables in independent samples and chi-square test for categorical variables. For non-parametric data, Mann-Whitney test was used for continuous variables in independent samples. Risk factors for having a medication discrepancy (dependent variable) were analyzed with Pearson correlation tests for parametric variables and with Spearman correlation tests for nonparametric variables. Correlations were calculated between number of discrepancies and the following variables: age, gender, number of comorbidities, Charlson Comorbidity Index, number of medications preadmission and number of clinical data sources. Only the values with strong correlations were reported (r≥0.6 or r≤-0.6). The significance threshold was .05, except for multiple comparisons performed using Bonferroni correction. A sample size of 65 patients in each group was calculated to have 80% power to detect a difference of 2 days in LHS in each group, assuming an expected standard deviation of 4.0 for a 2-sided t test with 5.0% type I error. Primary outcome analyses were performed in the intention-to-treat principle.
All analyses were conducted with Statistica 8.0 (StataSoft, Inc. 2007, Tulsa, USA).
RESULTS
During the six months of the study, 442 patients were admitted and classified as eligible (Figure 2). From this cohort, almost 75% were excluded for the following reasons: refusal to participate (n=26) and not matching inclusion criteria (n=283), younger than 18 years old (n=3), admission not occurring in a study unit (n=30), communication difficulties (n=48), transfer less than 72 hours after admission (n=86), and lack of a pharmacist to conduct the clinical interview (n=116). The randomization process assigned 68 patients to UC and 65 to MR (Figure 2). In UC, during the follow up, 3 patients were excluded due to serious discrepancies (omission of hypoglycemic agents requiring pharmacist intervention) and 19 were lost due to early transfer (n=17) and unrelated death (n=2). In the MR, 24 patients were lost by early transfer (n=23) and unrelated death (n=1). During the period of data analysis, 2 MR patients were excluded who, due to socioeconomic fragility, remained hospitalized for 122 days; according to the consistency analysis, they were considered outliers.
Figure 2.
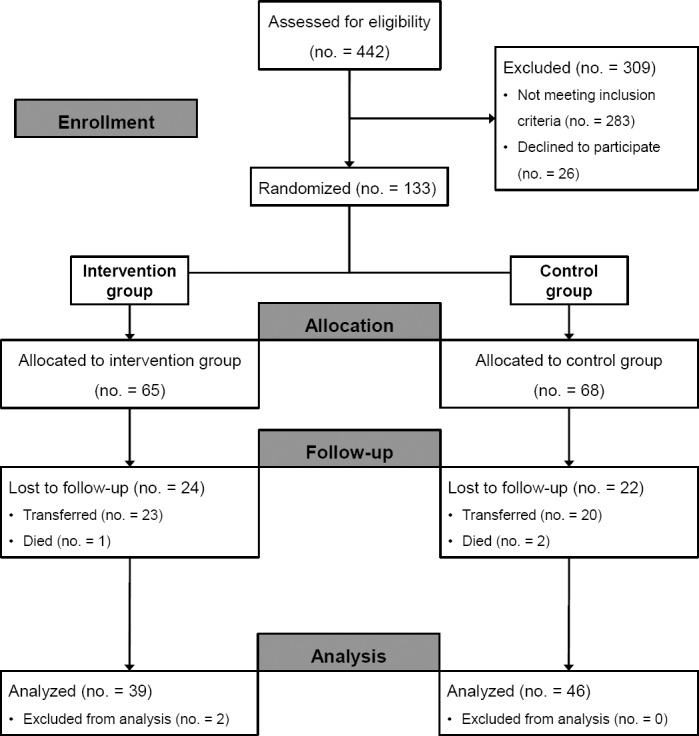
Study flow diagram
Similar to the study of Tompson et al.19 patients had a high prevalence of cardiovascular diseases (51.8%), followed by endocrine (41.2%), gastrointestinal (21.2%), hematologic/oncologic (20.0%), infectious diseases (10.6%), and pulmonary disorders (10.6%). Other groups of diseases were less prevalent and were displayed by fewer than 10.0% of the patients. All baseline characteristics (Table 1) as well comorbidities were similar in both groups (P>0.05). The mean of LHS was 10 (SD=15) days in the UC and 9 (SD=16) days in the MR (P=0.620). During medication history collection, there was a mean of 6 (SD=3) of preadmission medications in both groups (P=0.512). The number of medications increases at admission: 9 (SD=3) in the UC and 8 (SD=2) in the MR (P=0.313). Each patient in MR group had an average of 5 (SD=2) discrepancies.
Table 1.
Baseline sample characteristics
| Parameters | Total (n = 133) | Usual Care Group (n = 68) | Medication Reconciliation Group (n = 65) | p-value |
|---|---|---|---|---|
| Female, n (%) | 40 (47.1) | 22 (47.8) | 18 (46.1) | 0.878 |
| Age, mean (SD) | 53 (16) | 54 (15) | 53 (17) | 0.837 |
| Number of comorbidities, median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–4) | 0.607 |
| Charlson Comorbidity Index, mean (SD) | 2.9 (2.3) | 2.5 (2.3) | 3.4 (2.1) | 0.805 |
SD= standard deviation; IQR= interquartile range
Of the 327 discrepancies found in the MR, 172 (52.6%) were unintentional. Omission, dosage, interval, and route of administration, respectively, were the most common type of discrepancies found. More details about the interventions performed and the percentage of acceptance are described in Figure 3. As result of correlation analysis, the number of discrepancies was primarily explained by the following continuous variables: age (r=0.67, P=0.046), number of medications preadmission (r=0.78, P=0.021), and Charlson Comorbidity Index (r=0.61, P=0.038).
Figure 3.
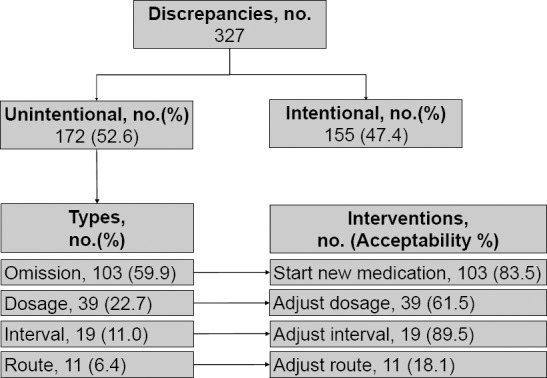
Discrepancies and Interventions in Medication Reconciliation Group
The mean time to perform MR was 40 (SD=17.2) minutes. In the evaluation of clinical data sources accessed to collect medication history, a median of two (2–4) sources were available for each patient, both for the total sample and groups. The main source of collected data was direct interview with the patient in MR group (Table 2).
Table 2.
Available data sources accessed to medication history in MR group
| Data sources per patient, median (IQR) | 2 (2–4) |
| Patient interview, n (%) | 39 (100.0) |
| Brown bag data, n (%) | 11 (28.2) |
| Preadmission order(s), n (%) | 13 (33.3) |
| Caregiver, n (%) | 12 (30,8) |
DISCUSSION
In the first Brazilian randomized controlled trial about MR at admission, correlations between age, use of medications, health condition, and the number of discrepancies after the collection of the medication history were found. This is evidence that such features can be used to create groups with higher risk of having discrepancies, making MR more effective.
The number of unintentional discrepancies was strongly correlated with age (r=0.67) and number of medications preadmission (r=0.78). This indicates that elderly patients who are polymedicated are more likely to present discrepancies, as previously published.20 Further, the correlation with the Charlson Comorbidity Index was significant (r=0.61), suggesting that the severity of the patient’s condition may lead to an increased number of discrepancies and thus to a higher risk of adverse events.
Medication errors are common at hospital admission.21,22 In this study, a significant number of unintentional discrepancies (n=3; SD=1 per patient) were found in MR group. This is consistent with other studies in which a high percentage of patients admitted showed several unintended discrepancies.8,22,23 Another relevant point is that, as in other studies24,25, about 64% of patients had at least one discrepancy at the time of hospital admission. This shows the importance of discrepancies for health care security, since most of the discrepancies concerning the list of pre-admission medications have the potential to cause harm and are clinically significant.7
Qualitative assessment of the discrepancies showed that, as in other studies7,8,22,23,26, omission is the most common type, representing 59.9% of the unintentional discrepancies. However, this finding is inconsistent with the study that ranks the discrepancies of route and interval as second and third places.23 The prevalence of polymedicated patients, with a mean of 6 (SD=3) medications in the pre-admission list, may explain omission as the major discrepancy, due to the difficulty of patients and caregivers to accurately inform pharmacotherapy at the time of admission.
As expected, suggesting the start of a medication was the most frequently performed intervention due to the amount of omission discrepancies found. However, suggesting the adjustment of interval was the most accepted intervention, with 89.5% of acceptance, then starting new medication (83.5%), adjusting the dosage (61.5%), and changing route (18.1%).
This study did not find significant differences in LHS between the UC and MR groups (P=0.620). This is inconsistent with the study in which admission discrepancies corrected within 48 hours by the pharmacist intervention caused a small but significant reduction in the LHS.19 The lack of outcome efficacy in this study may be related to sample heterogeneity, due to patients at age extremes and with great variation in the Charlson Comorbidity Index, even though there was no significant difference in the group comparison.
When assessing the data collection process of the intervention phase, the variables evaluated were number of data sources used in the collection phase and time spent to perform the intervention. In Beckett’s 2012 study27, pharmacists used a mean of 1.7 sources to collect the medication history; similar data was found in this trial with a median of 2 (1–3) sources per patient. A mean of 40.0 (SD=17.2) minutes were spent on time for intervention, about two times longer than indicated in Beckett’s study.27
The criteria for enrollment were comprehensive and focused on the availability of pharmacists to perform the proposed actions rather than classifying individuals specifically into a socio-demographic, health condition, or medication use group. Thus, the characteristics of the sample are close to those presented by the population of patients admitted to public Brazilian teaching hospitals, which allows extrapolation of the results obtained in this study.
Despite the existence of statistical homogeneity between groups, great variation in gender, age, number of preadmission medications used and Charlson Comorbidity Index parameters was found within the groups. Since in the same group there were patients with parameters ranging from 19–89 for age, 0–14 medications used in preadmission, and 0–8 for the Charlson Comorbidity Index, this is likely associated with the great variation within the groups for LHS. One limitation of our study was a considerable loss of follow-up due to transfer of patients. Substantial loss to follow-up can lead to underestimation of the true intervention effect. Another observed limitation was the small number of trained pharmacists who had time available to perform MR. As a consequence we were unable to perform MR among the first 48 hours after admission as recommended in several guidelines.10
CONCLUSIONS
These results highlight weakness at patient transition care levels in a public teaching hospital. LHS, the primary outcome, should be further investigated in larger studies. MR was well accepted by physicians and it is a useful tool to find and correct discrepancies, minimizing the risk of adverse drug events and improving patient safety.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest or financial disclosures relevant to this article were reported by any of the authors.
Contributor Information
Antonio E. Mendes, Internal Medicine Department, Federal University of Paraná, Curitiba (Brazil). mmendesantonio@gmail.com
Natália F. Lombardi, Pharmacy Department, Federal University of Paraná, Curitiba (Brazil). natalia.f.lombardi@gmail.com
Vânia S. Andrzejevski, Hospital Pharmacy Unit of Clinics Hospital, Federal University of Paraná. Curitiba (Brazil). salvivania@gmail.com
Gibran Frandoloso, Internal Medicine Department, Federal University of Paraná. Curitiba (Brazil). gibran.af@gmail.com.
Cassyano J. Correr, Pharmacy Department, Federal University of Paraná. Curitiba (Brazil). cassyano.correr@gmail.com
Mauricio Carvalho, Internal Medicine Department, Federal University of Paraná. Curitiba (Brazil). m_carvalho@hotmail.com.
References
- 1.Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358(10):1064–1071. doi: 10.1056/NEJMhpr0706165. doi:10.1056/NEJMhpr0706165. [DOI] [PubMed] [Google Scholar]
- 2.Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533–536. doi: 10.7326/0003-4819-141-7-200410050-00009. [DOI] [PubMed] [Google Scholar]
- 3.Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323. doi: 10.1002/jhm.228. [DOI] [PubMed] [Google Scholar]
- 4.Makaryus AN, Friedman EA. Patients’ understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994. doi: 10.4065/80.8.991. [DOI] [PubMed] [Google Scholar]
- 5.Calkins DR, Davis RB, Reiley P, Phillips RS, Pineo KL, Delbanco TL, Iezzoni LI. Patient-physician communication at hospital discharge and patients’ understanding of the postdischarge treatment plan. Arch Intern Med. 1997;157(9):1026–1030. [PubMed] [Google Scholar]
- 6.Pàez Vives F, Recha Sancho R, Altadill Amposta A, Montaña Raduà RM, Anadón Chortó N, Castells Salvadó M. An interdisciplinary approach to reconciling chronic medications on admission to Mora d’Ebre local hospital. Rev Calid Asist. 2010;25(5):308–313. doi: 10.1016/j.cali.2010.03.002. doi:10.1016/j.cali.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Pippins JR, Gandhi TK, Hamann C, Ndumele CD, Labonville SA, Diedrichsen EK, Carty MG, Karson AS, Bhan I, Coley CM, Liang CL, Turchin A, McCarthy PC, Schnipper JL. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–1422. doi: 10.1007/s11606-008-0687-9. doi:10.1007/s11606-008-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173(5):510–515. doi: 10.1503/cmaj.045311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman EA, Smith JD, Raha D, Min S. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842–1847. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 10.The Audit Commission. [accessed March 1 2015];A spoonful of sugar. Medicines management in NHS hospitals. [Internet] Available from: http://www.audit-commission.gov.uk/nationalstudies/health/other/Pages/aspoonfulofsugar.aspx .
- 11.Delgado Sánchez O, Anoz Jiménez L, Serrano Fabiá A, Nicolás Pico J. Conciliation in medication. Med Clin (Barc) 2007;129(9):343–348. doi: 10.1157/13109550. [DOI] [PubMed] [Google Scholar]
- 12.Paparella S. Medication reconciliation: doing what’s right for safe patient care. J Emerg Nurs. 2006;32(6):516–520. doi: 10.1016/j.jen.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Resources JC. Using medication reconciliation to prevent errors. Jt Comm J Qual Patient Saf. 2006;32(4):230–232. doi: 10.1016/s1553-7250(06)32030-2. [DOI] [PubMed] [Google Scholar]
- 14.Saufl NM. Reconciliation of medications. J Perianesth Nurs. 2006;21(2):126–127. doi: 10.1016/j.jopan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Manno MS, Hayes DD. Best-practice interventions: how medication reconciliation saves lives. Nursing. 2006;36(3):63–64. doi: 10.1097/00152193-200603000-00045. [DOI] [PubMed] [Google Scholar]
- 16.Rotta I, Salgado TM, Felix DC, Souza TT, Correr CJ, Fernandez-Llimos F. Ensuring consistent reporting of clinical pharmacy services to enhance reproducibility in practice: an improved version of DEPICT. J Eval Clin Pract. 2015;21(4):584–590. doi: 10.1111/jep.12339. doi:10.1111/jep.12339. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Dooley MJ, Allen KM, Doecke CJ, Galbraith KJ, Taylor GR, Bright J, Carey DL. A prospective multicentre study of pharmacist initiated changes to drug therapy and patient management in acute care government funded hospitals. Br J Clin Pharmacol. 2004;57(4):513–521. doi: 10.1046/j.1365-2125.2003.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tompson AJ, Peterson GM, Jackson SL, Hughes JD, Raymond K. Utilizing community pharmacy dispensing records to disclose errors in hospital admission drug charts. Int J Clin Pharmacol Ther. 2012;50(9):639–646. doi: 10.5414/CP201720. doi:10.5414/CP201720. [DOI] [PubMed] [Google Scholar]
- 20.Salanitro AH, Osborn CY, Schnipper JL, Roumie CL, Labonville S, Johnson DC, Neal E, Cawthon C, Businger A, Dalal AK, Kripalani S. Effect of patient- and medication-related factors on inpatient medication reconciliation errors. J Gen Intern Med. 2012;27(8):924–932. doi: 10.1007/s11606-012-2003-y. doi:10.1007/s11606-012-2003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duguid M. The importance of medication reconciliation for patients and practioners. Aust Prescr. 2012;35:15–19. [Google Scholar]
- 22.Cornish PL, Knowles SR, Marchesano R, Tam V, Shadowitz S, Juurlink DN, Etchells EE. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424–429. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]
- 23.Gleason KM, Groszek JM, Sullivan C, Rooney D, Barnard C, Noskin GA. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm. 2004;61(16):1689–1695. doi: 10.1093/ajhp/61.16.1689. [DOI] [PubMed] [Google Scholar]
- 24.Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15(2):122–126. doi: 10.1136/qshc.2005.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubowski TJ, Cronin LM, Pavelka RW, Briscoe-Dwyer LA, Briceland LL, Hamilton RA. Effectiveness of a medication reconciliation project conducted by PharmD students. Am J Pharm Educ. 2007;71(5):656. doi: 10.5688/aj710594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gleason KM, McDaniel MR, Feinglass J, Baker DW, Lindquist L, Liss D, Noskin GA. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25(5):441–447. doi: 10.1007/s11606-010-1256-6. doi:10.1007/s11606-010-1256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckett RD, Crank CW, Wehmeyer A. Effectiveness and feasibility of pharmacist-led admission medication reconciliation for geriatric patients. J Pharm Pract. 2012;25(2):136–141. doi: 10.1177/0897190011422605. doi:10.1177/0897190011422605. [DOI] [PubMed] [Google Scholar]


