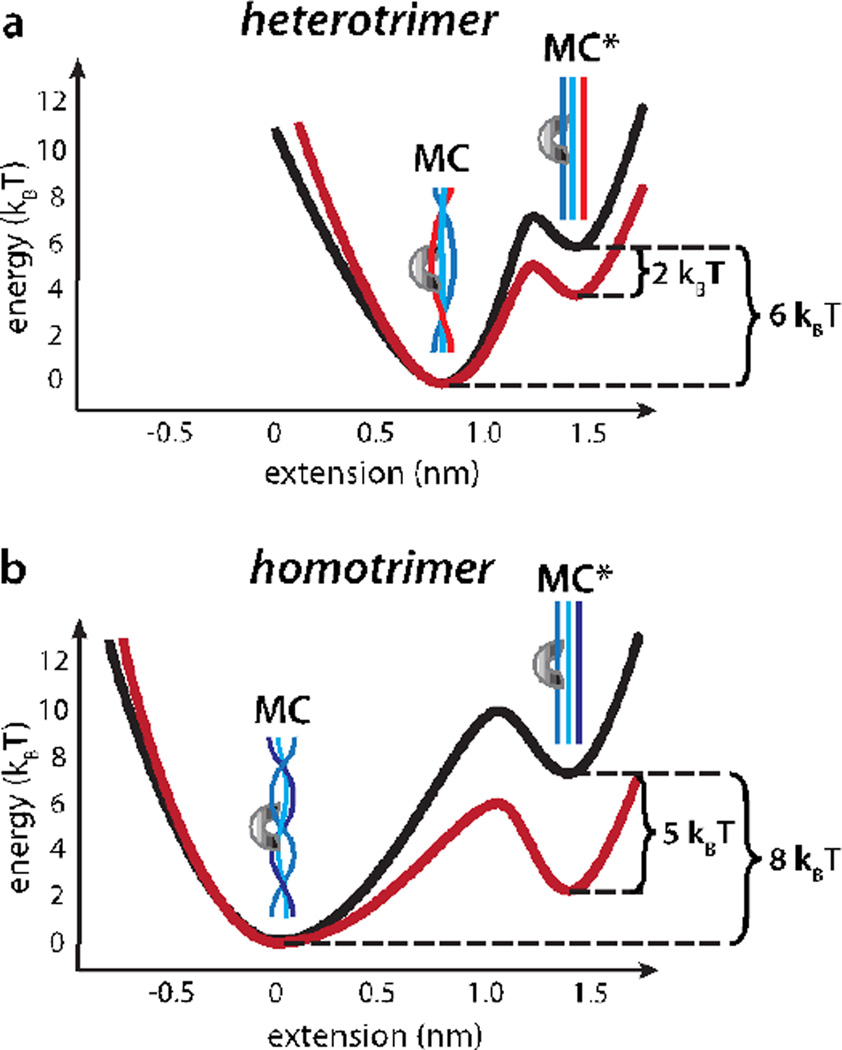
Figure 5.
Energetic landscape for collagen proteolysis by MMP-1. (a) The heterotrimeric MMP-1 binding site is partially unwound even in the absence of applied load. Approximately 6 times the thermal energy (6 kBT) separates the MC and MC* states (black; see text). A 0.57 nm increase in length accompanies the transition from MC to MC*. 15 pN external load stabilizes MC* by 8.6 pN·nm, or ~2 kBT (red). (b) Approximately 8 kBT separate MC and MC* for the homotrimeric MMP-1 recognition site (black; see text). A 1.4 nm length increase accompanies the transition to the stretched state, resulting in ~5 kBT stabilization of MC* at 15 pN of load (red).