Figure 2.
Hyperphosphorylation of FGFR and RAS-MAPK Activation in an Individual with ECCL Due to p.Asn546Lys Substitution
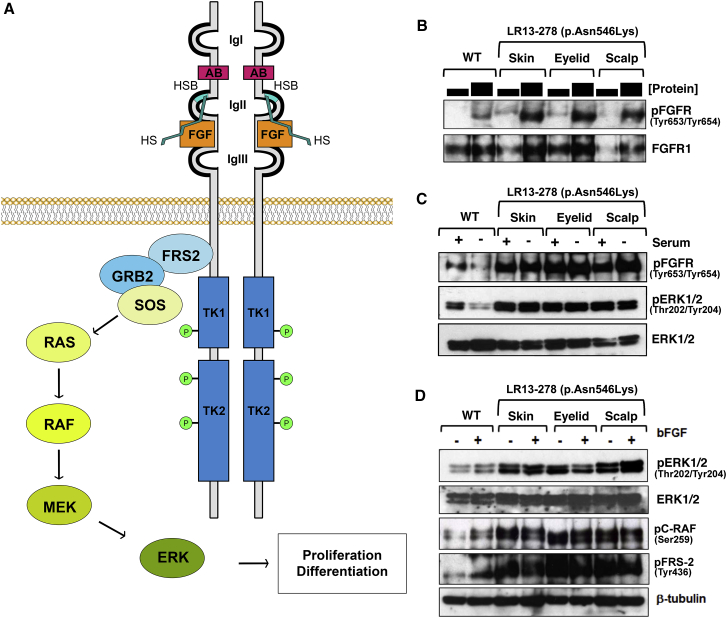
(A) Ligand and heparan-sulfate binding induces FGFR dimerization and conformational changes followed by trans-phosphorylation and activation of the cytoplasmic kinase domain. Phosphorylation of additional tyrosine sites in the kinase domain creates high affinity binding sites for downstream effector proteins such as FRS2, which recruits GRB2 and initiates RAS-MAPK signaling. The three extracellular Ig-like domains, the acid box (AB), heparan-sulfate (HS), heparan-sulfate binding site (HSB), fibroblast growth factor (FGF), and the two-part tyrosine kinase (TK1 and TK2) domain are shown.
(B) Differing amounts of whole-cell extract (WCE) from exponentially growing wild-type (WT) and ECCL fibroblasts derived from various tissues from LR13-278 were probed for FGFR-phosphorylation using pan-FGFR phosphorylation antibodies (pFGFR-Tyr653/Tyr654).
(C) WCE was prepared from exponentially growing cells (+ serum) and from cells that were serum starved for 72 hr (− serum) from wild-type (WT) fibroblasts and from various tissues from LR13-278. These were blotted using antibodies to detect pan-FGFR transphosphorylation and ERK1/2 phosphorylation.
(D) All fibroblasts were serum starved for 72 hr and then either untreated (−) or treated (+) with bFGF (10 nM for 15 min). WCE from wild-type (WT) and LR13-278 fibroblasts from various tissues were blotted to detect ERK1/2 and C-RAF phosphorylation and also for FRS2 phosphorylation. All antibodies were obtained from Cell Signaling Technology: anti-pFGFR-Tyr653/Tyr654 (Cat #3471S), anti-FGFR-1 (Cat #9740), anti-pFRS2-Tyr463 (Cat #3861S), anti-pERK1/2-Thr202/Tyr204 (Cat #9101S), anti-ERK1/2 (Cat #4695S), and anti-pC-RAF-Ser259 (Cat #9421).