Abstract
The purpose is to compare quantitative dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) metrics with imaging tumor size for early prediction of breast cancer response to neoadjuvant chemotherapy (NACT) and evaluation of residual cancer burden (RCB). Twenty-eight patients with 29 primary breast tumors underwent DCE-MRI exams before, after one cycle of, at midpoint of, and after NACT. MRI tumor size in the longest diameter (LD) was measured according to the RECIST (Response Evaluation Criteria In Solid Tumors) guidelines. Pharmacokinetic analyses of DCE-MRI data were performed with the standard Tofts and Shutter-Speed models (TM and SSM). After one NACT cycle the percent changes of DCE-MRI parameters Ktrans (contrast agent plasma/interstitium transfer rate constant), ve (extravascular and extracellular volume fraction), kep (intravasation rate constant), and SSM-unique τi (mean intracellular water lifetime) are good to excellent early predictors of pathologic complete response (pCR) vs. non-pCR, with univariate logistic regression C statistics value in the range of 0.804 to 0.967. ve values after one cycle and at NACT midpoint are also good predictors of response, with C ranging 0.845 to 0.897. However, RECIST LD changes are poor predictors with C = 0.609 and 0.673, respectively. Post-NACT Ktrans, τi, and RECIST LD show statistically significant (P < .05) correlations with RCB. The performances of TM and SSM analyses for early prediction of response and RCB evaluation are comparable. In conclusion, quantitative DCE-MRI parameters are superior to imaging tumor size for early prediction of therapy response. Both TM and SSM analyses are effective for therapy response evaluation. However, the τi parameter derived only with SSM analysis allows the unique opportunity to potentially quantify therapy-induced changes in tumor energetic metabolism.
Introduction
Neoadjuvant chemotherapy (NACT) is commonly used as standard of care treatment for locally advanced breast cancer with the primary clinical goals of downstaging the disease, improving operability, and allowing breast conserving surgery [1], [2]. Though NACT does not improve overall survival in comparison with postoperative adjuvant therapy [3], [4], [5], one advantage of NACT is to provide the opportunity for assessment of pathologic response to the treatment. Pathologic complete response (pCR) or minimal residual disease following NACT has been shown to be prognostic for survival [6], [7], [8], [9]. The majority of the patients, however, do not achieve pCR with the pCR rate reported in the range of 6% to 45% depending on breast cancer subtypes and treatment regimens [6], [7], [10], [11]. Furthermore, the pathologic response status is generally determined only from the surgical specimen after NACT completion. Therefore, there is a genuine and unmet need for reliable and minimally invasive imaging methods to provide early prediction of response to NACT. In the emerging era of precision medicine, early prediction of NACT response may allow rapid, personalized treatment regimen alterations for non-responding breast cancer patients and spare them from potential short and long term toxicities associated with ineffective therapies. Additionally, accurate evaluation of residual disease after NACT is vital for surgical decision making and could result in surgical treatment plans more tailored to individual patients.
Imaging tumor size change with guidelines such as RECIST (Response Evaluation Criteria In Solid Tumors) [12] is routinely used in clinical trial settings to assess tumor response to treatment. However, size change in response to therapy is often found to manifest later than changes in underlying tumor functions [13], [14], [15], [16], such as vascularization and vascular permeability, cellularity, and metabolism. Recognized as a minimally invasive imaging method for evaluation of perfusion and permeability, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is increasingly used in research and early phase clinical trial settings to measure and, importantly, predict tumor response to treatment [13], [14]. Over the last decade, substantial evidence has accumulated [17], [18], [19] showing the utility of DCE-MRI for assessment and early prediction of breast cancer response to NACT. Despite large variations in DCE-MRI data acquisition protocol details (temporal resolution, spatial resolution and coverage, acquisition time length, etc.) and data analysis methods (semi-quantitative vs. quantitative pharmacokinetic analysis of signal intensity time-course data), many studies have shown that changes in several semi-quantitative [20], [21], [22], [23], [24], [25] or quantitative [26], [27], [28], [29], [30], [31], [32], [33], [34], [35] DCE-MRI metrics during the course of NACT can provide good early prediction of pathologic response after one to two NACT cycles, and valuable clinical evaluation of overall response and prognosis. In correlating DCE-MRI parameters with pathologic response endpoints, most studies use binary discrimination of pCR and non-pCR with few [23] reporting relationships between post-NACT imaging metrics and pathologically measured residual disease burden, which could have important implications for surgical decision making. Among studies that performed quantitative pharmacokinetic analyses of DCE-MRI data, most employed the nuclear medicine, tracer kinetic model based Tofts model (TM) [36], [37] with inherent neglect of the effects of intercompartmental water exchange kinetics. The water molecule is not the signal molecule in nuclear medicine imaging, but in DCE-MRI it is. Considering the two-compartment model of intra- and extra-cellular spaces, for example, since contrast agent (CA) molecules generally reside in the extracellular space, the cross-cell membrane water exchange kinetics needs to be accounted for when converting MRI signal intensity change to tissue CA concentration change in pharmacokinetic analysis of DCE-MRI data.
In this paper we report our initial results in DCE-MRI assessment of breast cancer response to NACT. The DCE-MRI data were analyzed using both the TM and the Shutter-Speed model (SSM), which takes into account the finite intercompartmental water exchange kinetics [38], [39]. DCE-MRI parameters, including the SSM-unique τi parameter (mean intracellular water lifetime), an inverse measure of cellular metabolic activity [40], [41], were evaluated and compared between the TM and SSM and with imaging tumor size measurement, for early prediction of pathologic response to NACT and assessment of residual disease.
Materials and Methods
Patient Cohort and Study Schema
In this institutional review board–approved and HIPAA-compliant study, twenty-eight women who were diagnosed with 29 grade 2 to 3 invasive breast tumors (one patient had two independent primary tumors) and to undergo NACT were consented to participate in a longitudinal research MRI study that includes DCE-MRI. Twenty one (22 primary tumors) of the 28 patients were treated with standard of care therapy regimens that include four cycles of doxorubicin-cyclophosphamide every 2 weeks followed by four cycles of a taxane every 2 weeks, or six cycles of the combination of all three drugs every 3 weeks. The targeted agent, trastuzumab, was added to the regimen for tumors with positive HER2 (human epidermal growth factor receptor 2) receptor status. The other seven patients (seven primary tumors) were enrolled in the NACT ISPY-2 trial (http://ispy2.org), where patients were randomized to receive standard of care regimen or standard of care regimen plus experimental drugs. The ISPY-2 standard of care regimen starts with a taxane, followed by doxorubicin-cyclophosphamide. If used, the experimental drug is usually added to the taxane. Four of the seven patients were placed in the treatment arm with experimental drugs: three (patient 7, 12, and 13, Table 1) received neratinib, a tyrosine kinase inhibitor, and the other (patient 24) received ganitumab, a human monoclonal antibody against type 1 insulin-like growth factor receptor (IGF1R). The clinicopathologic characteristics of the studied patient cohort are presented in Table 1.
Table 1.
Clinicopathologic Characteristics of the Study Patient Cohort Treated with Neoadjuvant Chemotherapy
| Patient Number |
Age (yr) | Tumor Type | Tumor Grade | Pre-treatment Size (cm) |
Receptor Status(ER,PR,HER2) | Treatment Regimen | Pathologic Response | RCB Class |
|---|---|---|---|---|---|---|---|---|
| 1 | 27 | IDC | 2 | 3.7(Mammo) | −, −, + | Docetaxel + carboplatin + trastuzumab | non-pCR | III |
| 2 | 27 | IDC | 2 | 1.3(MRI) | +, +, + | Docetaxel + carboplatin + trastuzumab | pCR | 0 |
| 3 | 61 | IDC | 3 | 2.4(MRI) | −, −, − | Adriamycin + cyclophosphamide, then paclitaxel | non-pCR | I |
| 4 | 39 | IDC | 2 | 2.6(MRI) | +, +, + | Paclitaxel + trastuzumab, then cyclophosphamide + adriamycin | pCR | 0 |
| 5 | 63 | IDC | 2 | 2.3(US) | −, −, + | Docetaxel + carboplatin + trastuzumab | pCR | 0 |
| 6 | 56 | IDC | 3 | 4.4(US) | −, −, − | Paclitaxel, then cyclophosphamide + adriamycin | non-pCR | I |
| 7 | 62 | IDC | 3 | 2.1(Mammo) | −, −, − | Carboplatin + neratinib, then cyclophosphamide + adriamycin | non-pCR | II |
| 8 | 65 | IDC | 2 | 2.1(Mammo) | +, +, − | Cyclophosphamide + adriamycin, then paclitaxel | non-pCR | II |
| 9 | 46 | IDC | 2 | 2.5(Mammo) | +, +, − | Cyclophosphamide + adriamycin + docetaxel | non-pCR | II |
| 10 | 33 | IDC | 2 | 2.0(US) | +, +, + | Docetaxel + carboplatin + trastuzumab | non-pCR | II |
| 11 | 41 | IDC | 2 | 3.0(MRI) | +, +, - | Cyclophosphamide + adriamycin + docetaxel | non-pCR | II |
| 12 | 35 | IDC | 2 | 2.8(MRI) | −, +, + | Paclitaxel + neratinib, then cyclophosphamide + adriamycin | pCR | 0 |
| 13 | 39 | IDC | 3 | 2.7(MRI) | +, −, + | Paclitaxel + neratinib, then cyclophosphamide + adriamycin | non-pCR | I |
| 14 | 42 | IDC IDC |
2 2 |
1.6(US) 1.8(US) |
+, +, + +, −, − |
Doxorubicin + cyclophosphamide, then docetaxel + trastuzumab | non-pCR non-pCR |
II I |
| 15 | 34 | IDC | 2 | 5.0(MRI) | +, +, − | Paclitaxel, then cyclophosphamide + adriamycin | non-pCR | II |
| 16 | 45 | ILC | 2 | 11.8(MRI) | +, +, − | Paclitaxel, then cyclophosphamide + adriamycin | non-pCR | III |
| 17 | 38 | IDC | 3 | 3.6(US) | −, −, + | Docetaxel + carboplatin + trastuzumab | non-pCR | III |
| 18 | 59 | IDC | 3 | 2.0(US) | +, +, + | Docetaxel + carboplatin + trastuzumab | non-pCR | II |
| 19 | 46 | IDC | 3 | 1.7(US) | −, −, + | Docetaxel + carboplatin + trastuzumab | non-pCR | I |
| 20 | 59 | IDC | 2 | 3.0(MRI) | −, −, + | Paclitaxel + trastuzumab, then cyclophosphamide + adriamycin | pCR | 0 |
| 21 | 51 | IDC | 2 | 3.2(Mammo) | +, +, − | Cyclophosphamide + Adriamycin + docetaxel | non-pCR | I |
| 22 | 75 | ILC | 2 | 2.5(Mammo) | −, −, − | Adriamycin + cyclophosphamide, then paclitaxel | non-pCR | II |
| 23 | 34 | IDC | 3 | 2.1(MRI) | −, −, + | Adriamycin + cyclophosphamide, then paclitaxel + trastuzumab | non-pCR | I |
| 24 | 32 | IDC | 3 | 5.9(MRI) | +, +, − | Paclitaxel + ganitumab, then cyclophosphamide + adriamycin | non-pCR | II |
| 25 | 44 | IDC | 2 | 2.8(MRI) | +, +, − | Adriamycin + cyclophosphamide, then paclitaxel | non-pCR | I |
| 26 | 37 | IDC | 3 | 9.8(MRI) | −, −, − | Cyclophosphamide + Adriamycin + docetaxel | non-pCR | II |
| 27 | 48 | IDC | 3 | 2.9(Mammo) | −, +, − | Adriamycin + cyclophosphamide, then paclitaxel | non-pCR | III |
| 28 | 31 | IDC | 3 | 1.6(US) | −, +, − | Adriamycin + cyclophosphamide, then paclitaxel | non-pCR | I |
IDC: invasive ductal carcinoma; ILC: invasive lobular carcinoma; Pre-treatment size: imaging tumor size in the longest diameter before treatment; Mammo: mammography; US: ultrasound; ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; pCR: pathologic complete response; RCB: residual cancer burden.
The MRI exams for this research study were performed pre-NACT (Visit 1, V1), after one cycle of NACT (V2), at midpoint of NACT (V3; usually after 3 or 4 cycles of NACT, or before change of NACT agents), and after NACT completion but prior to surgery (V4). The ISPY-2 trial includes a different MRI protocol conducted at the same four time points. For the 7 patients who participated in the ISPY-2 trial, care was taken to ensure there were at least 24 hours between the research and ISPY-2 MRI studies to allow CA, used in both, clearance from the body. For the V2 – V4 studies, the MRI scan was undertaken at least 7 days after the administration of the previous NACT cycle to allow time for drug effects.
DCE-MRI Data Acquisition
All breast MRI studies were performed using a 3 T Siemens Tim Trio system with the body coil and a four-channel bilateral phased-array breast coil as the transmitter and receiver, respectively. In each MRI session, following pilot scans and pre-CA axial T2-weighted MRI with fat-saturation and axial T1-weighted MRI without fat-saturation, axial bilateral DCE-MRI images with fat-saturation (using the approach of water excitation only) and full breast coverage were acquired with a 3D gradient echo-based TWIST (Time-resolved angiography WIth Stochastic Trajectories) sequence, which employs the strategy of k-space undersampling during acquisition and data sharing during reconstruction [42]. Compared to conventional full k-space sampling gradient echo sequences, the TWIST sequence enables acceleration of image acquisition and preserves signal-to-noise ratio, while the image artifacts can be minimized using appropriate k-space undersampling and view sharing strategies [42]. DCE-MRI acquisition parameters included 10o flip angle, 2.9/6.2 ms TE/TR, a parallel imaging acceleration factor of 2, 30 to 34 cm FOV, 320×320 in-plane matrix size, and 1.4 mm slice thickness. The total acquisition time for a DCE-MRI series was ~ 10 minutes for 28 to 38 image volume sets of 96 to 128 slices each with 14.6 to 20.2 s temporal resolution. The variations in number of image volumes, number of slices per volume, and temporal resolution were due to differences in breast size. The intravenous injection of the CA, Gd(HP-DO3A) [ProHance (Bracco Diagnostic Inc.)] (0.1 mmol/kg at 2 mL/s), by a programmable power injector was timed to commence after acquisition of two baseline image volumes, followed by a 20-mL saline flush.
For quantification of the pre-CA T1 value, T10, proton density-weighted images were acquired immediately before and spatially co-registered with the DCE-MRI scan [42], [43]. The data acquisition sequence and parameters were the same as for the DCE-MRI scan except for 5° flip angle and 50 ms TR.
DCE-MRI Data Analysis
Breast tumor regions of interest (ROIs) were drawn by two experienced breast radiologists on post-CA (approximately 90 to 120 s after the CA injection) multi-slice DCE images covering the entire contrast-enhanced tumor. They also measured the longest diameter (LD) of the tumor from these images using the RECIST guidelines [12]. To avoid within-subject inter-observer variations, all images from the longitudinal study of a given patient were interpreted by one radiologist.
For the purpose of pharmacokinetic modeling of DCE-MRI data, the tumor ROI- and voxel-based T10 value was determined by comparing signal intensities between the proton density-weighted images and the baseline images from the DCE series [42], [43]. The ROI-averaged and voxel (within the tumor ROI) DCE-MRI time-course data were separately fitted with different pharmacokinetic models: once with the one-compartment-two-parameter TM [36] and once with the two-compartment-three-parameter fast-exchange-regime (FXR)–allowed SSM [38]. Equations (1), (2) represent the pharmacokinetic time-course expressions for the TM and FXR-SSM, respectively:
| (1) |
| (2) |
where R1(t) is the tissue longitudinal relaxation rate constant, Cp(t′) is the arterial plasma CA concentration time course, or arterial input function (AIF), R1i is the intrinsic intracellular longitudinal relaxation rate constant, R10 (= 1/T10) is the pre-CA tissue R1, r1 is the tissue CA relaxivity, Ktrans is the rate constant for CA plasma-to-interstitium transfer, ve is the volume fraction of extravascular and extracellular space, and τi is the mean intracellular water molecule lifetime. Both model fittings return Ktrans and ve parameter values, and the CA intravasation rate constant, kep, can be calculated as kep = Ktrans/ve. However, only the SSM fitting returns the τi parameter. Since the TM neglects the finite intercompartmental water exchange kinetics, assuming the water exchange MR system is always in the fast-exchange-limit condition, the linear relationship between R1(t) and tissue CA concentration, Ct(t), shown in Equation (3) and implicit in Equation (1), is used:
| (3) |
The SSM accounts for the finite water exchange kinetics during the CA passage through the tissue of interest, and consequently R1(t) is not linearly related to Ct(t). The FXR-SSM takes into account transcytolemmal water exchange kinetics in the two-compartment model of intra- and extra-cellular compartments (in the extravascular space), but assumes single exponential longitudinal MR signal decay [38], [39], [41], [44].
A population-averaged AIF was used for pharmacokinetic analysis of DCE-MRI data for each patient and each visit. This AIF was obtained by averaging individually measured AIFs from an axillary artery in a previous sagittal breast DCE-MRI study with higher temporal resolution [44], which employed the same CA injection protocol, including dose, injection rate, and injection vein (antecubital vein). Following the TM and SSM fittings of the DCE-MRI data, voxel-based parametric maps of the derived pharmacokinetic parameters were generated. The mean whole tumor pharmacokinetic parameter value estimated with each model at each visit was calculated as the average of single-slice ROI parameter values from the multiple image slices covering the whole tumor, weighted by the number of voxels in each ROI. For each imaging metric, including pharmacokinetic parameters and RECIST LD, the percent changes for later visits relative to V1, V21% (V2 relative to V1), V31%, and V41%, were calculated.
Pathological Analysis
The status of pathologic response (to NACT) for each breast tumor was determined by pathological analysis of the post-NACT resection specimen. The pathology parameters measured from the resection specimen under light microscopy include: cross sectional tumor size in two dimensions (d1, d2, measured [in mm] grossly and confirmed microscopically), estimated invasive tumor cellular density (finv), number of involved lymph nodes (LN), and the greatest tumor dimension (dmet) in the largest involved node. The Residual Cancer Burden (RCB) index value was calculated using Equation (4) published by Symmans et al [9]:
| (4) |
A complete pathologic response (pCR) is defined as the absence of residual invasive tumor (RCB = 0). A pathologic non-response (pNR) is defined as tumor cell density in resection specimen equal to or greater than that in core biopsy specimen. Pathologic partial response (pPR) is defined as findings intermediate between pCR and pNR. Non-pCR includes both pPR and pNR and can be further stratified into RCB classes based on RCB index values [9]: RCB-I: 0 < RCB ≤ 1.36; RCB-II: 1.36 < RCB ≤ 3.28; RCB-III: RCB > 3.28. Since the MRI metrics were measured from the primary breast tumor only, the in-breast component of RCB (the first term on the right hand side of Equation (4)) [23] was also computed for correlation with the MRI results. The same RCB index value ranges were used for defining in-breast RCB classes.
Statistical Analysis
Descriptive statistical analysis was conducted to summarize the pharmacokinetic parameter (returned by each model fitting) and RECIST LD values at each visit, as well as the percent changes of these imaging metrics relative to baseline (V1). In assessing the ability for early prediction of therapy response, the univariate logistic regression (ULR) analysis was used to correlate V1, V2, V3 MRI metrics, and the corresponding V21% and V31% changes, with dichotomous pathologic response endpoints, pCR vs. non-pCR. A ULR C statistics value, a measure equivalent to the area under the receiver operating characteristic curve, in the range of 0.9 to 1.0 indicates an excellent predictor; 0.8 to 0.9, a good predictor; 0.7 to 0.8, a fair predictor; < 0.7, a poor predictor. A two-sample t test was used to evaluate the differences in imaging metrics and the corresponding percent changes between the pCR and non-pCR groups. In assessing the ability for evaluation of RCB (and in-breast RCB) following NACT completion, the ULR analysis was used to estimate the capabilities of V4 and V41% MRI metrics for discriminating RCB (and in-breast RCB) class, while the Spearman correlation (SC) analysis was used to correlate V4 and V41% MRI metrics with RCB (and in-breast RCB) index values.
Results
As shown in Table 1, pathological analyses of the surgical specimens revealed that 5 patients (5 primary tumors) achieved pCR following NACT, while the other 23 patients (24 primary tumors) all had pPR, or non-pCR. The RCB class for each tumor is also presented in Table 1.
Early Prediction of Pathologic Response
Table 2 lists the mean ± SD whole tumor MRI metric values of the pCR and non-pCR groups and the corresponding ULR C statistics values for early prediction of pCR vs. non-pCR. Only the absolute pharmacokinetic parameters and the V21% and V31% changes with C ≥ 0.8, representing good to excellent early predictors, are listed. The C values for V21% and V31% RECIST LD changes are presented for comparison. The V21% changes in tumor Ktrans(TM), Ktrans(SSM), kep(TM), and τi provide excellent (C > 0.9) early discrimination of pCR and non-pCR, while V2 and V3 ve estimated from either pharmacokinetic model, and their corresponding V21% and V31% changes, are good (0.8 < C < 0.9) markers for early prediction of response. The V21% and V31% changes in RECIST LD, however, are poor (C < 0.7) early predictors of response. Except for the RECIST LD changes, the differences in all other listed metrics between the two response groups are statistically significant (P < .05) or approaching significance. Other than τi at V1, which is a good early predictor of response with a C value of 0.826, no other pre-NACT MRI metrics have a C value greater than 0.7. Figure 1A shows the mean ± SD column graph of V21% changes in Ktrans(TM), Ktrans(SSM), kep(TM), ve(TM), ve(SSM), τi, and RECIST LD for the pCR and non-pCR groups. Note the substantially larger differences in the pharmacokinetic parameters between the two groups compared to RECIST LD. Figure 1B shows a similar graph for absolute TM and SSM ve values of the two patient groups at V2 and V3.
Table 2.
Early Prediction of Pathologic Response (pCR vs. non-pCR)
| MRI Metric |
pCR |
non-pCR |
ULR C value |
|
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | P value | ||
| V21% Ktrans(TM) | − 64% ± 9% | − 14% ± 41% | .098 | 0.967 |
| V21% kep(TM) | − 77% ± 9% | − 20% ± 44% | .050 | 0.957 |
| V21% Ktrans(SSM) | − 71% ± 9% | − 16% ± 50% | .052 | 0.957 |
| V21% τi | 41% ± 26% | − 11% ± 25% | .018 | 0.946 |
| V2 ve(SSM) | 0.78 ± 0.10 | 0.60 ± 0.14 | .073 | 0.897 |
| V21% ve(TM) | 80% ± 60% | 35% ± 42% | .026 | 0.880 |
| V21% ve(SSM) | 72% ± 41% | 19% ± 28% | .033 | 0.880 |
| V2 ve(TM) | 0.70 ± 0.37 | 0.29 ± 0.11 | .018 | 0.864 |
| V3 ve(TM) | 0.63 ± 0.31 | 0.32 ± 0.15 | .035 | 0.845 |
| V3 ve(SSM) | 0.81 ± 0.11 | 0.63 ± 0.15 | .088 | 0.845 |
| V1 τi (s) | 0.53 ± 0.16 | 0.81 ± 0.26 | .047 | 0.826 |
| V31% ve(SSM) | 80% ± 54% | 27% ± 30% | .041 | 0.810 |
| V21% kep(SSM) | − 77% ± 12% | − 11% ± 94% | .092 | 0.804 |
| V31% ve(TM) | 141% ± 115% | 65% ± 85% | .070 | 0.804 |
| V31% RECIST LD | − 35% ± 21% | − 26% ± 20% | .438 | 0.673 |
| V21% RECIST LD | − 15% ± 16% | − 10% ± 11% | .320 | 0.609 |
ULR: univariate logistic regression; SD: standard deviation; P value: two-sample t test; TM: Tofts model; SSM: Shutter-Speed model.
Figure 1.
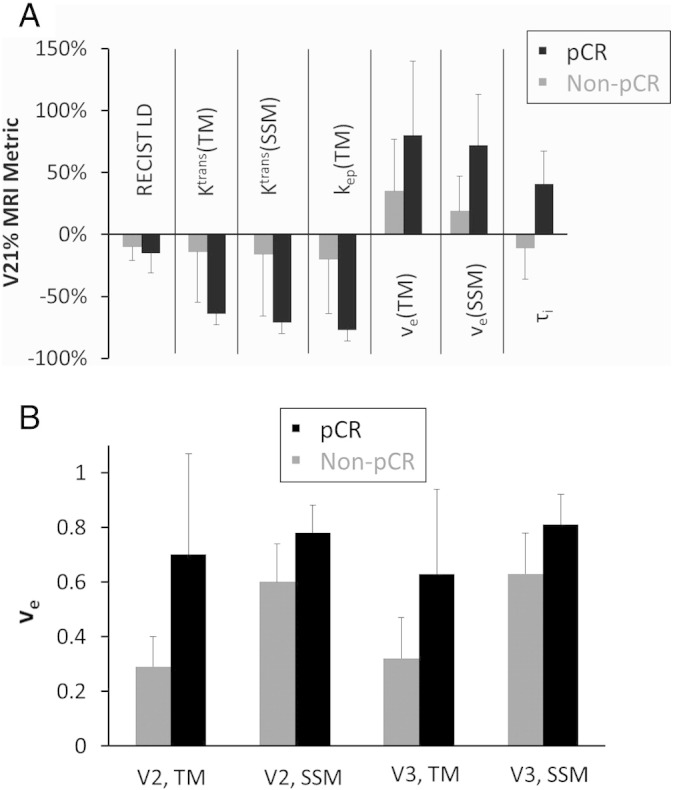
Column graphs of the (A) mean V21% change values of RECIST LD and several DCE-MRI metrics (Ktrans, ve, kep, and τi, estimated from the TM and SSM pharmacokinetic analyses) and (B) mean V2 and V3 ve values (TM and SSM) for the pCR (black column) and non-pCR (gray column) patient groups. The error bar represents the standard deviation (SD). V21%: percent change of MRI metric at visit 2 (V2, after one NACT cycle) relative to visit 1 (V1, pre-NACT); V3: visit 3, midpoint of NACT.
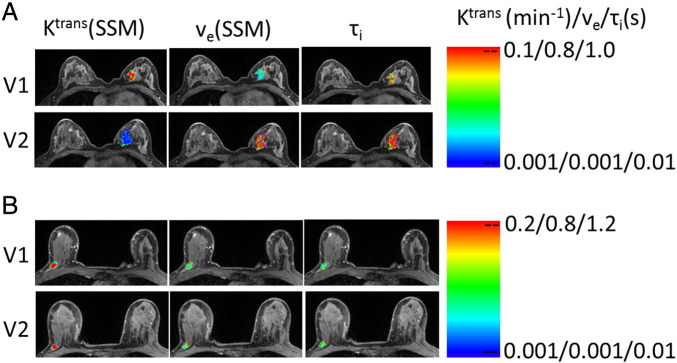
To demonstrate differences in early changes of tumor pharmacokinetic parameters following NACT initiation, Figure 2 shows examples of Ktrans(SSM), ve(SSM), and τi maps at V1 and V2 for a pCR (Figure 2A, patient 12) and a non-pCR (Figure 2B, patient 3) patient. The color tumor parametric maps are in image slices approximately through the center of the tumor in every case, and the color scales are kept the same through the two visits for each tumor. There are no noticeable changes in the three parametric maps from V1 to V2 for the non-pCR, while the considerable decrease in Ktrans(SSM) and increases in ve(SSM) and τi are clearly visible for the pCR.
Figure 2.
V1 (pre-NACT) and V2 (after one NACT cycle) color parametric maps of Ktrans(SSM), ve(SSM), and τi from a pCR (A, left breast, patient 12) and a non-pCR (B, right breast, patient 3) breast tumor. The maps were generated for tumor ROIs defined on multiple image slices, and the ones on the image slice through the central portion of the tumor are displayed here. For each tumor, the color scale of each DCE-MRI metric is kept the same between the two visits for easy visualization of NACT-induced changes.
MRI evaluation of RCB after NACT
Table 3A presents the ULR C statistics values of several post-NACT MRI metrics for differentiating RCB and in-breast RCB classes of 0, I, II, and III. Only the metrics with C ≥ 0.7 (fair or better markers of RCB class) for in-breast RCB classes are listed, which include absolute values of V4 Ktrans(SSM), Ktrans(TM), kep(SSM), kep(TM), τi, and RECIST LD. The discriminative abilities of these metrics are improved slightly when in-breast RCB class is used in place of RCB class. There is not a single V41% change metric that is at least a fair marker of RCB or in-breast RCB class.
Table 3A.
Discrimination of Post-NACT RCB Class
| MRI Metric⁎ | ULR C value |
|
|---|---|---|
| RCB class | In-breast RCB class | |
| V4 Ktrans(SSM) | 0.801 (0.680, 0.922) | 0.837 (0.734, 0.940) |
| V4 Ktrans(TM) | 0.797 (0.669, 0.925) | 0.833 (0.708, 0.958) |
| V4 τi | 0.783 (0.647, 0.919) | 0.792 (0.634, 0.950) |
| V4 RECIST LD | 0.727 (0.586, 0.868) | 0.732 (0.538, 0.870) |
| V4 kep(SSM) | 0.697 (0.540, 0.854) | 0.719 (0.520, 0.890) |
| V4 kep(TM) | 0.694 (0.553, 0.835) | 0.705 (0.527, 0.857) |
Imaging results are missing from a pCR patient (patient 20), who declined the V4 MRI study due to personal reasons. The values in the parentheses are 95% confidence intervals (CIs).
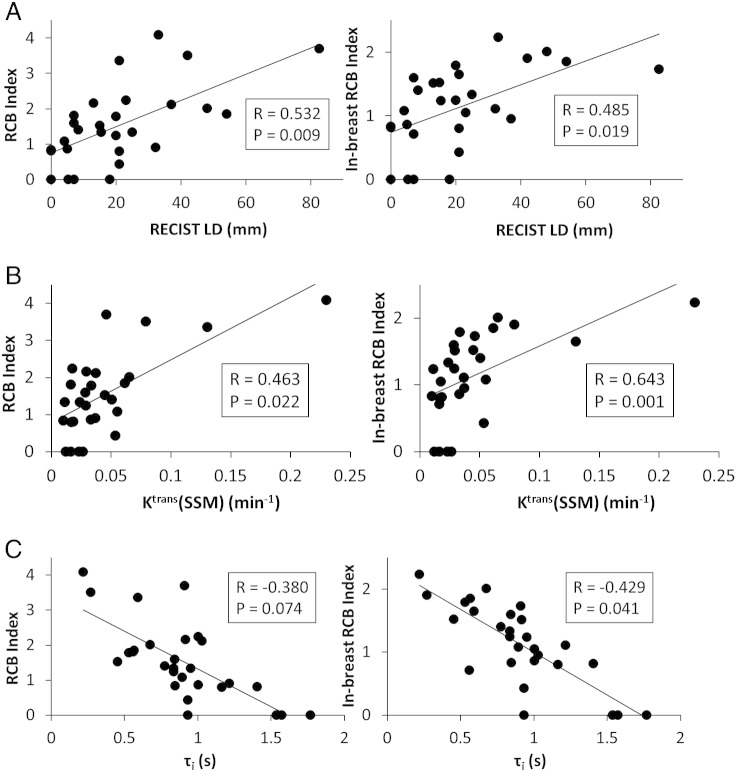
The SC coefficient, R, and the P value for statistical significance are summarized in Table 3B for correlation between RCB (and in-breast RCB) index value and post-NACT MRI metric value. Only those metrics with P < 0.1 are listed. As is the case of correlation with RCB class, the strength of SC is generally improved slightly when the in-breast RCB index value is used, and no V41% metric has a SC P value smaller than 0.1. The positive correlations between RCB and V4 RECIST LD, Ktrans(SSM), and Ktrans(TM) are statistically significant (P < .05) for both RCB and in-breast RCB values. The inverse correlation between RCB and V4 τi is significant (P = .041) for the in-breast RCB, while approaching significance (P = .074) for the RCB value. The positive correlation between RCB and V4 kep(SSM) is near statistical significance for both the RCB and in-breast RCB values. Figure 3 shows examples of linear regressions between the RCB (and in-breast RCB) index values and the V4 MRI metrics of RECIST LD (Figure 3A), Ktrans(SSM) (Figure 3B), and τi (Figure 3C).
Table 3B.
Spearman Correlation of Post-NACT RCB Index Value with MRI Metric Value
| MRI Metric⁎ | RCB |
In-breast RCB |
||
|---|---|---|---|---|
| R | P | R | P | |
| V4 RECIST LD | 0.532 | .009 | 0.485 | .019 |
| V4 Ktrans(SSM) | 0.463 | .022 | 0.643 | .001 |
| V4 Ktrans(SM) | 0.463 | .022 | 0.618 | .002 |
| V4 τi | − 0.380 | .074 | − 0.429 | .041 |
| V4 kep(SSM) | 0.366 | .078 | 0.376 | .070 |
R: Spearman correlation coefficient; P < 0.05 indicates statistically significant correlation.
Imaging results are missing from a pCR patient (patient 20), who declined the V4 MRI study due to personal reasons.
Figure 3.
Scatter plots of pathologically measured RCB and in-breast RCB index values (from post-NACT resection specimens) against post-NACT (V4) MRI metrics: (A) RECIST LD, (B) Ktrans(SSM), and (C) τi. The straight line in each panel represents a linear regression. The Spearman correlation coefficient R and P values for the three imaging metrics are listed in Table 3B and shown in each panel. Note the inverse relationship between RCB (and in-breast RCB) and τi. Imaging results are missing from a pCR patient (patient 20), who declined the V4 MRI study due to personal reasons.
Discussion
Consistent with several previous studies [26], [27], [28], [29], [30], [31], [32], [33], [34], [35] using DCE-MRI to assess breast cancer response to NACT, our initial findings from this study of a 28-patient cohort show that changes in tumor functions as measured by quantitative DCE-MRI are considerably more reliable early predictors of pCR compared to changes in imaging tumor size after only one of six or eight cycles of NACT. This suggests that therapy-induced tumor functional changes precede changes in tumor size. The percent changes of the Ktrans, ve, and kep parameters, as well as the SSM-unique τi parameter, are good to excellent early predictors of pathologic response. Additionally, the absolute values of ve after one NACT cycle (V2) or at NACT midpoint (V3) are also good early predictors of response. Imaging tumor size measurement under the RECIST guidelines is the current standard of care for evaluation of tumor response to treatment. However, our results reveal that changes in RECIST LD after one NACT cycle, or even at midpoint of NACT (Table 2), are poor early predictors of response. For example, under the condition of 100% sensitivity for prediction of pCR (i.e., correctly classify all five pCRs in the study cohort), the specificities are 92% and 17% for V21% Ktrans(TM) and V21% RECIST LD, respectively, meaning misclassification of only two out of 24 non-pCR tumors as pCRs when using V21% Ktrans(TM) as the imaging metric versus 20 out of 24 non-pCRs as pCRs when using V21% RECIST LD as the imaging metric. The ability of minimally invasive imaging parameters, such as the DCE-MRI parameters, to accurately provide early prediction of therapy response may have profound importance in the emerging era of precision and personalized medicine. Early identification of non-responders to a therapy regimen may allow rapid decision making in adjusting the treatment plan, e.g., changing drugs and/or undergoing surgery early, to spare these patients from the morbidity caused by ineffective and toxic chemotherapy agents. This will have significant positive impact on healthcare cost savings and patient wellbeing. Using this cohort as an example, if V21% Ktrans(TM) had been used as the imaging marker for early prediction of therapy response in clinical care, 22 out of 24 non-pCRs would have been correctly classified after only one NACT cycle and could have been treated with different therapy regimens or stratified for novel therapy trials.
Accurate imaging assessment of RCB after NACT can lead to better staging for surgery and more informed decision making in breast conservation surgery versus mastectomy. Though RECIST LD is not a good early predictor of pathologic response, its value measured after NACT completion (V4) is indicative of the RCB class and index value (Table 3A, Table 3B; Figure 3A). The post-NACT DCE-MRI parameters that are fair to good markers of RCB class and correlate significantly with RCB index value include Ktrans (estimated from either of the two models) and τi. Unsurprisingly, since the MRI metrics were measured from the primary tumor only, the correlations with RCB are slightly improved when the in-breast RCB class and value are used (Table 3A, Table 3B; Figure 3). With a larger patient cohort, meaningful multivariate analysis may be performed to potentially identify a combination of imaging metrics including both tumor size and DCE-MRI functional parameters that can provide even more accurate measure of RCB.
Since cytotoxic drugs such as doxorubicin, cyclophosphamide, and paclitaxel were used throughout the NACT regimen for each patient studied, it is to be expected that increases in ve after one NACT cycle or at NACT midpoint are found to be good early predictors of response. The therapy-induced cancer cell death presumably leads to decrease in the extravascular, intracellular volume fraction, vi (≡ 1 – ve), which is dominated by decrease in cell density [41], and hence increase in ve. However, at the early stage of NACT a decrease in vi is not necessarily associated with a decrease in perfused tumor area, or contrast-enhanced MRI tumor size, the basis for RECIST LD measurement. This hypothesis on why the ve percent change is a good early predictor of response while the RECIST LD percent change is a poor predictor needs to be further tested in future studies. On the other hand, the post-NACT (V4) RECIST LD is a better measure of RCB than ve. This could be due to the fact that the RCB calculation (Equation (4)) includes dominant contribution from the product of cross sectional tumor sizes d1 and d2 measured from the resection specimen, while the association of vi (or 1 – ve) with finv is not as distinct - the former measures overall cell density [41] while the latter characterizes invasive cancer cell density.
The percent deceases in Ktrans and kep after one NACT cycle are good to excellent early predictors of response, suggesting these imaging metrics of perfusion and permeability may be sensitive parameters for prediction of NACT response. The therapy-induced changes in these microvascular properties are probably due to NACT secondary effects [14], since none of the standard of care or experimental drugs used in this cohort is known to be antiangiogenic. It has been suggested [45] that cytotoxic chemotherapy agents may affect tumor vasculature by interfering with endothelial cell function without causing endothelial cell death or interfering with a specific portion of the angiogenic cascade. The results from this study and others [26], [27], [28], [29], [30], [31], [32], [33], [34] indicate that it is the early changes in Ktrans and kep, not the perfused tumor area as measured by RECIST LD, that are good predictors of therapy response. This observation supports the hypothesis that the chemotherapeutics effects tumor vasculature without causing endothelial cell death [45]. One interesting finding is that though the V31% change in ve is still a good early predictor of response (Table 2), the V31% changes in Ktrans and kep are degraded to only fair predictors of response (not shown). For example, V31% Ktrans(TM) and V31% Ktrans(SSM) have ULR C values of 0.756 and 0.750, respectively. The decrease in the predictive ability is because the pCRs have such larger decreases in Ktrans and kep at V2 compared to non-pCRs that, by V3 (NACT midpoint), the percent changes (relative to baseline, V31%) in these metrics of the non-pCRs, though still smaller, draw near to those of the pCRs. Therefore, it is important to detect early microvascular changes with DCE-MRI, as they are better indicators of complete response to NACT than later changes.
The τi parameter is used to account for the effects of transcytolemmal water exchange kinetics and unique to the SSM method. A recent NMR spectroscopy study of yeast cell suspension [40] shows that the reciprocal of τi, kio (≡ 1/τi), the first-order rate constant for equilibrium cellular water efflux, is positively associated with cellular ATP levels. The in vivo association of cellular ATP decrease with kio decrease was demonstrated by a DCE-MRI and 31P MR spectroscopy study of a murine melanoma model treated with lonidamine [46]. A series of enzymatic and genetic manipulations on cell suspensions, perfused tissue, in vivo animal models, and human data have shown that kio measures the homeostatic turnover of the cell membrane Na+,K+-ATPase [NKA] [41]. Previously, it has not been possible to measure homeostatic NKA activity in vivo. In this study the V21% change in breast tumor τi is an excellent early predictor of pathologic response (Table 2). The pCRs show significant increase in τi (or decrease in kio) compared to non-pCRs after one NACT cycle, consistent with a substantial decrease in tumor metabolic activities early after NACT initiation being generally a good indicator of complete response. It is interesting to note that the pCR group has smaller pre-NACT (V1) τi values and thus greater metabolic turnover than the non-pCR group (Table 2), and V1 τi is the best predictor of response (a good metric with ULR C = 0.826) of all the V1 MRI metrics. The post-NACT (V4) τi is well correlated with the RCB index value in an inverse relationship (Table 3B and Figure 3C). These findings suggest that, over the course of the entire NACT regimen, the good responders have greater increases in τi, or decreases in metabolic activity, than the poor responders. In fact, from V1 to V4 the mean τi value of the pCR group is increased from 0.53 to 1.45 s, while that of the non-pCR group remains stable (0.81 to 0.82 s). The importance of τi as an imaging parameter for evaluation of breast cancer response to NACT is further confirmed by a pre-clinical DCE-MRI study of a genetically engineered mouse model of human breast tumor [47], which shows τi increase with almost no vi change following treatment with an experimental targeted, non-cytotoxic drug.
The DCE-MRI data acquired in this study were analyzed with both the TM and SSM. For the three parameters that can be estimated with both models, Ktrans, ve, and kep, the percent changes of these parameters in the early stage of NACT and the absolute values after NACT have similar capabilities for early prediction of response and evaluation of RCB, respectively, when comparing the two models. This is likely due to the fact that the underestimation of the Ktrans and ve parameters in malignant tumors by the TM relative to the SSM is generally systematic [27], [44]. The systematic parameter variations between the two models were largely cancelled in percent change calculation, or caused parameter values of each tumor to shift in the same direction when estimated from one pharmacokinetic model or the other. As a result, TM and SSM analyses performed equally well in early prediction and evaluation of therapy response. Li et al. [28] reported similar findings for early prediction of breast cancer response to NACT. Nonetheless, water exchange across tissue compartments is a real physiological phenomenon. Therefore, the SSM analysis approach should be used when T1-weighted DCE-MRI data are acquired with a protocol that is sensitive to water exchange, which usually also results in better signal-to-noise ratio [48]. Furthermore, the SSM analysis allows estimation of the metabolic activity imaging metric kio, adding a metabolic dimension to DCE-MRI, which is conventionally considered only as a functional imaging method for assessment of tissue microvasculature. As discussed above, the τi (or kio− 1) parameter is a very good marker for early prediction of response and accurate assessment of residual disease. The ability to characterize synergistic microvascular properties and cellular energetic metabolism simultaneously may harbor great promise for SSM DCE-MRI as an imaging tool to study the tumor microenvironment, and its response to treatment.
This study has several major limitations. First, with only 29 primary breast tumors, the sample size of the study cohort is small. The discriminative ability of a metric tends to be overestimated when the sample size is small. This is possibly the reason why the ULR C values of several DCE-MRI metrics obtained in this study for early prediction of breast cancer response to NACT are greater than the equivalent area under the receiver operating characteristic curve values reported by similar studies with larger cohort sizes [22], [23], [26], [28], [29]. Thus, it is important to validate the initial findings from this cohort with a larger patient population, especially for new findings specific to this study such as changes in ve and τi as early predictors of response. Second, as a result of small sample size, the imaging results are not stratified by receptor status-based breast cancer molecular subtypes in correlation with pathology endpoints. The number of patients is simply not adequate to draw meaningful conclusions in early discrimination of pCR and non-pCR or evaluation of RCB for each breast tumor subtype, and make comparisons between the subtypes. As such, the initial findings reported here reflect the average results from a general breast cancer population undergoing NACT. With continuing subject accrual, we will be able to examine and compare the utility of DCE-MRI for evaluation of therapy response in each breast cancer subtype in the future. Additionally, the small sample size also precludes meaningful multivariate analysis of the MRI metrics for further improvement in assessing therapy response. Third, mean tumor MRI metric values were used in this study for correlation with the pathology endpoints. It is well known that malignant tumors are heterogeneous in nature [41] and responses to treatment are likely heterogeneous as well. However, the heterogeneity in breast tumor functional changes in response to NACT was not captured in computing mean DCE-MRI parameter values. Recent studies show that texture analysis of tumor heterogeneity manifest in either raw image data [49], [50], [51] or parametric maps of kinetic features [52], [53] can be a valuable tool for evaluation of breast cancer therapy response. With voxel-based parametric maps of DCE-MRI metrics already generated in this study, texture analysis of these maps could potentially be performed. Measurement and integration of changes in both mean values and texture features of DCE-MRI metrics may further improve the robustness of quantitative DCE-MRI for assessment of therapy response.
In conclusion, in this DCE-MRI study of 29 primary breast tumors undergoing NACT, we have shown that changes in quantitative functional MRI metrics estimated by either TM or SSM analysis of DCE-MRI data provide substantially better early prediction of pathologic response to NACT than changes in imaging tumor size as measured by RECIST LD. Along with post-NACT RECIST LD, several post-NACT DCE-MRI parameters are good markers of RCB. The TM and SSM analyses perform equally well for early prediction of response and evaluation of RCB. However, the SSM method provides additional assessment of tumor metabolic activity changes in response to NACT, and the SSM-unique τi parameter is the only pre-therapy MRI metric that provides good early prediction of response. Future translation of quantitative DCE-MRI into clinical practice may help facilitate personalized treatment regimens for individual breast cancer patients and more informed decision making in breast conservation surgery vs. mastectomy.
Acknowledgements
The authors thank the breast cancer patients who voluntarily participated in this research study and Mr. William Woodward for assistance in breast DCE-MRI data acquisition.
Footnotes
Financial Support: This study was supported by NIH grants U01-CA154602 and R44-CA180425.
References
- 1.Hayes DF, Schott AF. Neoadjuvant chemotherapy: what are the benefits for the patient and for the investigator? J Natl Cancer Inst Monogr. 2015;2015:36–39. doi: 10.1093/jncimonographs/lgv004. [DOI] [PubMed] [Google Scholar]
- 2.Schott AF, Hayes DF. Defining the benefits of neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:1747–1749. doi: 10.1200/JCO.2011.41.3161. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–2685. doi: 10.1200/JCO.1998.16.8.2672. [DOI] [PubMed] [Google Scholar]
- 4.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 5.Redden MH, Fuhrman GM. Neoadjuvant chemotherapy in the treatment of breast cancer. Surg Clin North Am. 2013;93:493–499. doi: 10.1016/j.suc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Bonnefoi H, Litiere S, Piccart M, MacGrogan G, Fumoleau P, Brain E, Petit T., Rouanet P., Jassem J., Moldovan C. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol. 2014;25:1128–1136. doi: 10.1093/annonc/mdu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B., Eiermann W., Hilfrich J., Huober J. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 8.Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favorable prognosis for breast cancer patients. Eur J Cancer. 2011;47:2084–2090. doi: 10.1016/j.ejca.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 9.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 11.Zambetti M, Mansutti M, Gomez P, Lluch A, Dittrich C, Zamagni C, Ciruelos E, Pavesi L, Semiglazov V, De Benedictis E. Pathological complete response rates following different neoadjuvant chemotherapy regimens for operable breast cancer according to ER status, in two parallel, randomized phase II trials with an adaptive study design (ECTO II) Breast Cancer Res Treat. 2012;132:843–851. doi: 10.1007/s10549-011-1660-6. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, Nation Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor JPB, Jackson A, Parker GJM, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012;9:167–177. doi: 10.1038/nrclinonc.2012.2. [DOI] [PubMed] [Google Scholar]
- 14.Leach MO, Morgan B, Tofts PS, Buckley DL, Huang W, Horsfield MA, Chenevert TL, Collins DJ, Jackson A, Lomas D. Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur Radiol. 2012;22:1451–1464. doi: 10.1007/s00330-012-2446-x. [DOI] [PubMed] [Google Scholar]
- 15.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010;256:348–364. doi: 10.1148/radiol.10091760. [DOI] [PubMed] [Google Scholar]
- 16.Harry VN, Semple SI, Parkin DE, Gilbert FJ. Use of new imaging techniques to predict tumor response to therapy. Lancet Oncol. 2010;11:92–102. doi: 10.1016/S1470-2045(09)70190-1. [DOI] [PubMed] [Google Scholar]
- 17.Lobbes MBI, Prevos R, Smidt M, Tjan-Heijnen VCG, van Goethem M, Schipper R, Beets-Tan RG, Wildberger JE. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013;4:163–175. doi: 10.1007/s13244-013-0219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinovich ML, Sardanelli F, Ciatto S, Mamounas E, Brennan M, Macaskill P, Irwig L, von Minckwitz G, Houssami N. Early prediction of pathologic response to neoadjuvant chemotherapy in breast cancer: systematic review of the accuracy of MRI. Breast. 2012;21:669–677. doi: 10.1016/j.breast.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Wu LM, Hu JN, Gu HY, Hua J, Chen J, Xu JR. Can diffusion-weighted MR imaging and contrast-enhanced MR imaging precisely evaluate and predict pathological response to neoadjuvant chemotherapy in patients with breast cancer? Breast Cancer Res Treat. 2012;135:17–28. doi: 10.1007/s10549-012-2033-5. [DOI] [PubMed] [Google Scholar]
- 20.Pickles MD, Lowry M, Manton DJ, Turnbull LW. Prognostic value of DCE-MRI in breast cancer patients undergoing neoadjuvant chemotherapy: a comparison with traditional survival indicators. Eur Radiol. 2015;25:1097–1106. doi: 10.1007/s00330-014-3502-5. [DOI] [PubMed] [Google Scholar]
- 21.Woolf DK, Padhani AR, Taylor NJ, Gogbashian A, Li SP, Beresford MJ, Ah-See ML, Stirling J, Collins DJ, Makris A. Assessing response in breast cancer with dynamic contrast-enhanced magnetic resonance imaging: are signal intensity-time curves adequate? Breast Cancer Res Treat. 2014;147:335–343. doi: 10.1007/s10549-014-3072-x. [DOI] [PubMed] [Google Scholar]
- 22.Abramson RG, Li X, Hoyt TL, Su PF, Arlinghaus LR, Wilson KJ, Abramson VG, Chakravarthy AB, Yankeelov TE. Early assessment of breast cancer response to neoadjuvant chemotherapy by semi-quantitative analysis of high-temporal resolution DCE-MRI: preliminary results. Magn Reson Imaging. 2013;31:1457–1464. doi: 10.1016/j.mri.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hylton NM, Blume JD, Bernreuter WK, Pisano ED, Rosen MA, Morris EA, Weatherall PT, Lehman CD, Newstead GM, Polin S. Locally advanced breast cancer: MR imaging for prediction of response to neoadjuvant chemotherapy – results from ACRIN 6657/I-SPY trial. Radiology. 2012;263:663–672. doi: 10.1148/radiol.12110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansen R, Jensen LR, Rydland J, Goa PE, Kvistad KA, Bathen TF, Axelson DE, Lundgren S, Gribbestad IS. Predicting survival and early clinical response to primary chemotherapy for patients with locally advanced breast cancer using DCE-MRI. J Magn Reson Imaging. 2009;29:1300–1307. doi: 10.1002/jmri.21778. [DOI] [PubMed] [Google Scholar]
- 25.Martincich L, Montemurro F, De Rosa G, Marra V, Ponzone R, Cirillo S, Gatti M, Biglia N, Sarotto I, Sismondi P. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83:67–76. doi: 10.1023/B:BREA.0000010700.11092.f4. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Kang H, Arlinghaus LR, Abramson RG, Chakravarthy AB, Abramson VG, Farley J, Sanders M, Yankeelov TE. Analyzing spatial heterogeneity in DCE- and DW-MRI parametric maps to optimize prediction of pathologic response to neoadjuvant chemotherapy in breast cancer. Transl Oncol. 2014;7:14–22. doi: 10.1593/tlo.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang W, Li X, Chen Y, Li X, Chang MC, Oborski MJ, Malyarenko DI, Muzi M, Jajamovich GH, Fedorov A. Variations of dynamic contrast-enhanced magnetic resonance imaging in evaluation of breast cancer therapy response: a multicenter data analysis challenge. Transl Oncol. 2014;7:153–166. doi: 10.1593/tlo.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Arlinghaus LR, Ayers GD, Chakravarthy AB, Abramson RG, Abramson VG, Atuegwu N, Farley J, Mayer IA, Kelley MC. DCE-MRI analysis methods for predicting the response of breast cancer to neoadjuvant chemotherapy: pilot study findings. Magn Reson Med. 2014;71:1592–1602. doi: 10.1002/mrm.24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tateishi U, Miyake M, Nagaoka T, Terauchi T, Kubota K, Kinoshita T, Daisaki H, Macapinlac HA. Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging—prospective assessment. Radiology. 2012;263:53–63. doi: 10.1148/radiol.12111177. [DOI] [PubMed] [Google Scholar]
- 30.Li SP, Makris A, Beresford MJ, Taylor NJ, Ah-See MLW, Stirling JJ, d’Arcy JA, Collins DJ, Kozarski R, Padhani AR. Use of dynamic contrast-enhanced MR imaging to predict survival in patients with primary breast cancer undergoing neoadjuvant chemotherapy. Radiology. 2011;260:68–78. doi: 10.1148/radiol.11102493. [DOI] [PubMed] [Google Scholar]
- 31.Jensen LR, Garzon B, Heldahl MG, Bathen TF, Lundgren S, Gribbestad IS. Diffusion-weighted and dynamic contrast-enhanced MRI in evaluation of early treatment effects during neoadjuvant chemotherapy in breast cancer patients. J Magn Reson Imaging. 2011;34:1099–1109. doi: 10.1002/jmri.22726. [DOI] [PubMed] [Google Scholar]
- 32.Ah-See MLW, Makris A, Taylor NJ, Harrison M, Richman PI, Burcombe RJ, Stirling JJ, d’Arcy JA, Collins DJ, Pittam MR. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2008;14:6580–6589. doi: 10.1158/1078-0432.CCR-07-4310. [DOI] [PubMed] [Google Scholar]
- 33.Yankeelov TE, Lepage M, Chakravarthy A, Broome EE, Niermann KJ, Kelley MC, Meszoely I, Mayer IA, Herman CR, McManus K. Integration of quantitative DCE-MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging. 2007;25:1–13. doi: 10.1016/j.mri.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padhani AR, Hayes C, Assersohn L, Powles T, Makris A, Suckling J, Leach MO, Husband JE. Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical results. Radiology. 2006;239:361–374. doi: 10.1148/radiol.2392021099. [DOI] [PubMed] [Google Scholar]
- 35.Pickles MD, Lowry M, Menton DJ, Gibbs P, Turnbull LW. Role of dynamic contrast enhanced MRI in monitoring early response of locally advanced breast cancer to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2005;91:1–10. doi: 10.1007/s10549-004-5819-2. [DOI] [PubMed] [Google Scholar]
- 36.Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. Magn Reson Med. 1991;17:357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- 37.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ. Estimating kinetic parameters from dynamic contrast-enhanced T1-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 38.Yankeelov TE, Rooney WD, Li X, Springer CS. Variation of the relaxographic “Shutter-Speed” for transcytolemmal water exchange affects the CR bolus-tracking curve shape. Magn Reson Med. 2003;50:1151–1169. doi: 10.1002/mrm.10624. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Rooney WD, Springer CS. A unified pharmacokinetic theory for intravascular and extracellular contrast agents. Magn Reson Med. 2005;54:1351–1359. doi: 10.1002/mrm.20684. [Erratum. Magn Reson Med 2006;55:1217.] [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Poirier-Quinot M, Springer CS, Balschi JA. Active trans-plasma membrane water cycling in yeast is revealed by NMR. Biophys J. 2011;101:2833–2842. doi: 10.1016/j.bpj.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Springer CS, Li X, Tudorica LA, Oh KY, Roy N, Chui SYC, Naik AM, Holtorf ML, Afzal A, Rooney WD. Intratumor mapping of intracellular water lifetime: metabolic images of breast cancer? NMR Biomed. 2014;27:760–773. doi: 10.1002/nbm.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tudorica LA, Oh KY, Roy N, Kettler MD, Chen Y, Hemmingson SL, Afzal A, Grinstead JW, Laub G, Li X. A feasible high spatiotemporal resolution breast DCE-MRI protocol for clinical settings. Magn Reson Imaging. 2012;30:1257–1267. doi: 10.1016/j.mri.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang W, Wang Y, Panicek DM, Schwartz LH, Koutcher JA. Feasibility of using limited-population-based average R10 for pharmacokinetic modeling of osteosarcoma dynamic contrast-enhanced MRI data. Magn Reson Imaging. 2009;27:852–858. doi: 10.1016/j.mri.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang W, Tudorica LA, Li X, Thakur SB, Chen Y, Morris EA, Tagge IJ, Korenblit M, Rooney WD, Koutcher JA. Discrimination of benign and malignant breast lesions by using shutter-speed dynamic contrast-enhanced MR imaging. Radiology. 2011;261:394–403. doi: 10.1148/radiol.11102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller KD, Sweeney CJ, Sledge GW. Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol. 2001;19:1195–1206. doi: 10.1200/JCO.2001.19.4.1195. [DOI] [PubMed] [Google Scholar]
- 46.Nath K, Paudyal R, Nelson DS, Pickup S, Zhou R, Leeper DB, Heitjan DF, Springer CS, Poptani H, Glickson JD. Acute changes in cellular-interstitial water exchange rate in DB-1 melanoma xenografts after lonidamine administration as a marker of tumor energetics and ion transport. Proc Intl Soc Magn Reson Med. 2014;22:2757. [Google Scholar]
- 47.Springer CS, Li X, Jayatilake ML, Pike MM, Rooney WD, Sears RC, Huang W. Metabolic imaging of early tumor therapy. Proc Intl Soc Magn Reson Med. 2015;23:3860. [Google Scholar]
- 48.Li X, Huang W, Rooney WD. Signal-to-noise ratio, contrast-to-noise ratio, and pharmacokinetic modeling considerations in dynamic-contrast-enhanced magnetic resonance imaging. Magn Reson Imaging. 2012;30:1313–1322. doi: 10.1016/j.mri.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed A, Gibbs P, Pickles M, Turbull L. Texture analysis in assessment and prediction of chemotherapy response in breast cancer. J Magn Reson Imaging. 2013;38:89–101. doi: 10.1002/jmri.23971. [DOI] [PubMed] [Google Scholar]
- 50.Teruel JR, Heldahl MG, Goa PE, Pickles M, Lundgren S, Bathen TF, Gibbs P. Dynamic contrast-enhanced MRI texture analysis for pretreatment prediction of clinical and pathological response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. NMR Biomed. 2014;27:887–896. doi: 10.1002/nbm.3132. [DOI] [PubMed] [Google Scholar]
- 51.Parikh J, Selmi M, Charles-Edwards G, Glendenning J, Ganeshan B, Verma H, Mansi J, Harries M, Tutt A, Goh V. Changes in primary breast cancer heterogeneity may augment midtreatment MR imaging assessment of response to neoadjuvant chemotherapy. Radiology. 2014;272:100–112. doi: 10.1148/radiol.14130569. [DOI] [PubMed] [Google Scholar]
- 52.Golden DI, Lipson JA, Telli ML, Ford JM, Rubin DL. Dynamic contrast-enhanced MRI-based biomarkers of therapeutic response in triple-negative breast cancer. J Am Med Inform Assoc. 2013;20:1059–1066. doi: 10.1136/amiajnl-2012-001460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashraf A, Gaonkar B, Mies C, DeMichele A, Rosen M, Davatzikos C, Kontos D. Breast DCE-MRI kinetic heterogeneity tumor markers: preliminary associations with neoadjuvant chemotherapy response. Transl Oncol. 2015;8:154–162. doi: 10.1016/j.tranon.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]