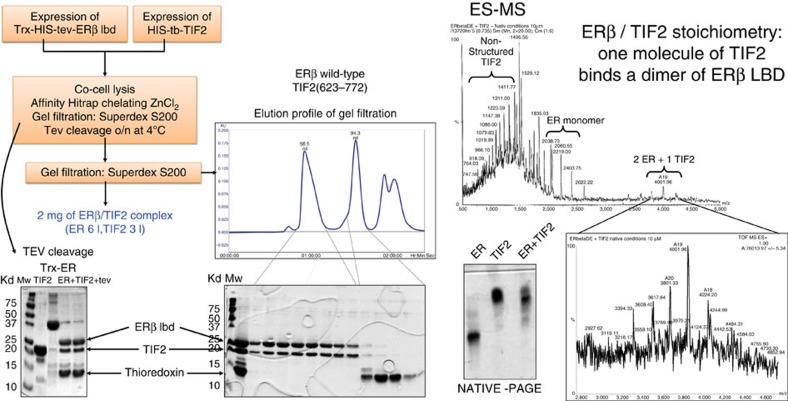
Figure 3. ERβ EF/TIF2 (623–772) complex purification and characterization.
The two partners were produced separately. The complex was formed by co-cell lysis followed by tag cleavage and purified by size exclusion chromatography. The thioredoxin tag was well separated from the complex as shown in the SDS–PAGE on the gel filtration elution fractions. The complex was analysed and characterized by native gel electrophoresis and ES-MS. The ES-MS spectrum revealed the presence of unfolded TIF2 (623–772) (poly-charged species), monomers of ERβ LBD and dimers of ERβ LBD bound to a folded monomer of TIF2 (623–772) suggesting an induced folding mechanism upon TIF2 binding.