Figure 3.
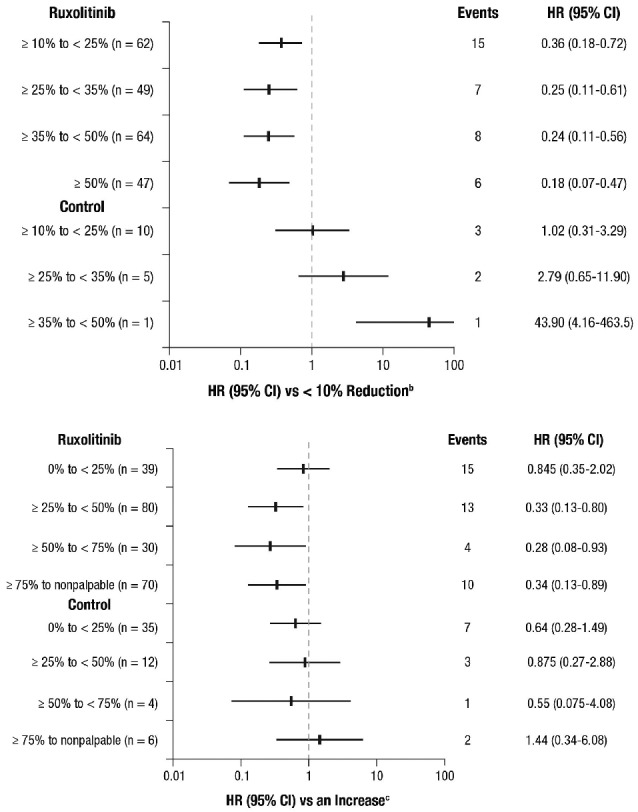
Correlation of (A) spleen volume reduction and (B) spleen length reduction at week 24 with overall survival (landmark analysis at 24 weeksa). aIncludes patients known to be alive at week 24. bCategory includes patients with a <10% reduction from baseline in spleen volume at week 24 or no assessment (ruxolitinib, n=64; control, n=189); among these patients, there were 26 deaths (events) in the pooled ruxolitinib group and 63 deaths in the control group. cCategory includes patients with no change or an increase from baseline in palpable spleen length at week 24 or no assessment (ruxolitinib, n=23; control, n=95); among these patients, there were 8 deaths (events) in the pooled ruxolitinib group and 28 deaths in the control group. HR: hazard ratio.
