FIG 10.
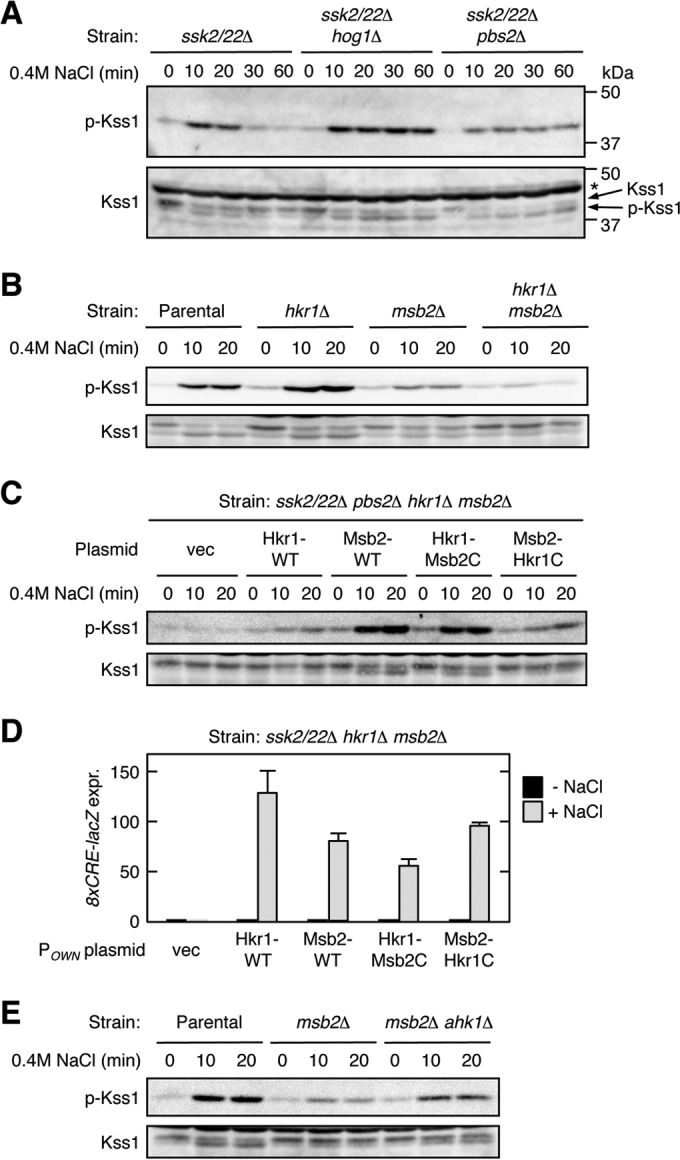
The Hkr1 cytoplasmic region suppresses HOG-FG cross talk. (A to C) Kss1 phosphorylation in response to osmostress. Exponentially growing cells were collected at the indicated times after addition of 0.4 M NaCl to the cultures, and the amounts of phosphorylated Kss1 (p-Kss1) and total Kss1 (Kss1) were determined by immunoblotting of the whole-cell lysate (20 μg protein per lane). Strains used were as follows: (A) TM257 (ssk2/22Δ), FP20 (ssk2/22Δ hog1Δ), and KT207 (ssk2/22Δ pbs2Δ); (B) KT207 (ssk2/22Δ pbs2Δ) (parental strain), KT071 (ssk2/22Δ pbs2Δ hkr1Δ), KT037 (ssk2/22Δ pbs2Δ msb2Δ), and KT074 (ssk2/22Δ pbs2Δ hkr1Δ msb2Δ); and (C) KT074 (ssk2/22Δ pbs2Δ hkr1Δ msb2Δ), which was transformed with the indicated plasmids. vec, empty vector; *, nonspecific band. (D) Expression of the Hog1-specific reporter gene 8xCRE-lacZ. The yeast strain KT063 (ssk2/22Δ hkr1Δ msb2Δ) was transformed with single-copy plasmids that expressed the indicated Hkr1 and Msb2 constructs from their native promoter (POWN is either PHKR1 or PMSB2, which corresponds to the 5′ end of the cloned gene), together with a reporter plasmid. Cells were stimulated with 0.4 M NaCl for 30 min or not stimulated, and expression of the 8xCRE-lacZ gene was determined. β-Galactosidase activity is expressed in Miller units. Error bars represent SD (n ≥ 3). (E) Kss1 phosphorylation in response to osmostress was assayed as described for panel A. Strains used were FP20 (ssk2/22Δ hog1Δ) (parental strain), TA124 (ssk2/22Δ hog1Δ msb2Δ), and AN33 (ssk2/22Δ hog1Δ msb2Δ ahk1Δ).
