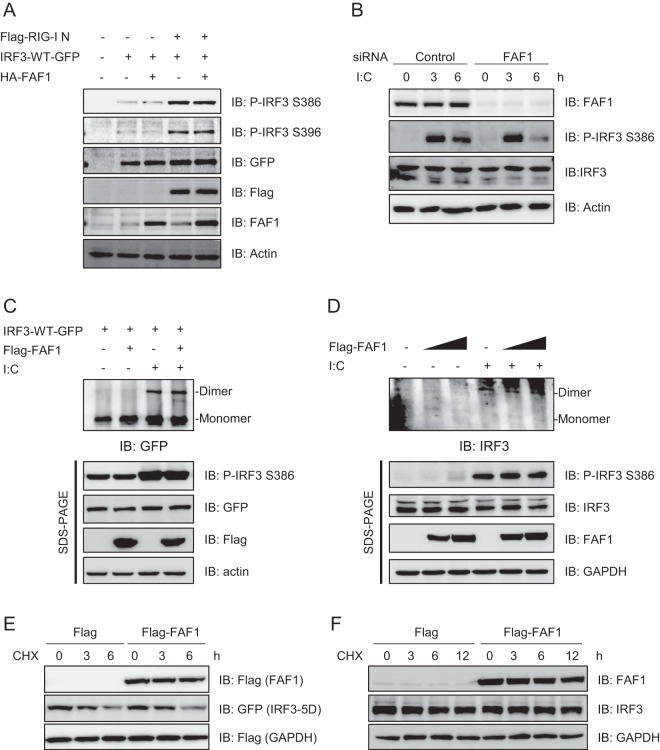
FIG 6.
FAF1 does not affect the phosphorylation and dimerization of IRF3. (A) HeLa cells were transfected with Flag-RIG-I N, IRF3-WT-GFP, HA-FAF1, or a control plasmid as indicated. At 24 h posttransfection, cell extracts were analyzed by Western blotting. Actin bands represent loading controls. (B) HeLa cells were transfected with a control siRNA or FAF1 siRNA 2 for 72 h and treated with poly(I·C) (10 μg/ml) by transfection. Cells were then harvested at the indicated time points, and extracts were analyzed by Western blotting, with actin bands representing loading controls. (C) HEK293T cells were cotransfected with IRF3-WT-GFP, Flag-FAF1, or a control plasmid and then stimulated with poly(I·C) (10 μg/ml) for 12 h. Cell extracts were separated by native gel or SDS-PAGE, and IRF3 dimers were detected by Western blotting, with actin bands representing loading controls. (D) HEK293T cells were transfected with Flag-FAF1 or a control plasmid and stimulated with poly(I·C) (10 μg/ml) for 12 h. Endogenous IRF3 was analyzed as described for panel C. (E) HeLa cells were transfected with Flag-FAF1, Flag-GAPDH, IRF3-5D-GFP, or a control plasmid. After 24 h, cells were treated with cycloheximide (50 μg/ml) for the indicated times. Cell lysates were separated by SDS-PAGE and analyzed by Western blotting. (F) HeLa cells were transfected with Flag or Flag-FAF1. After 24 h, cells were treated with cycloheximide (50 μg/ml) for the indicated times. The level of endogenous IRF3 was analyzed by Western blotting, with GAPDH bands representing loading controls.