Abstract
This study examined the feasibility of using event-related potentials (ERPs) to measure changes in cortical processing following an established rehabilitative intervention (constraint-induced movement therapy, CIMT) for children with cerebral palsy (CP). Sixteen participants with a diagnosis of hemiparetic CP, with a median age of 6 years, were assessed pre and immediately post CIMT and at 6-month follow-up, using a picture–word match/mismatch discrimination task and standard neurobehavioral measures. Intervention effects were evident in improved performance on behavioral tests of sensory and motor function and the increased mean ERP amplitude of the N400 match/mismatch response on the side ipsilateral to the lesion. These effects were maintained 6 months after the intervention. No such changes were observed on the side contralateral to the lesion. This research suggests that ERPs can measure rehabilitation-induced changes in neural function in children with CP.
Keywords: Cerebral palsy, Rehabilitation, Event-related potentials, Pediatric, Constraint therapy
The study of changes in neural connectivity after pediatric rehabilitation is essential to the understanding and design of evidence-based approaches to new designs. However, functional neuroimaging can be difficult when dealing with young children, especially those with disabilities. Transcranial magnetic stimulation (TMS; Berweck et al., 2006; Hamzei, Liepert, Dettmers, Weiller, & Rijntjes, 2006; Staudt et al., 2002), paired with magnetoencephalography and functional magnetic resonance imaging (fMRI), provides valuable mechanistic information (Juenger et al., 2007; Van de Winckel et al., 2013); however, this methodology requires lengthy protocols, is only effective with highly cooperative patients, and is not portable. While neuroimaging and neurobehavioral measures are essential to study the effects and mechanisms of pediatric rehabilitation, it may prove useful to develop a complementary approach to study changes in patterns of neural activation after therapy.
Event-related potentials (ERPs) are a portion of the ongoing electroencephalogram (EEG) time-locked to specific stimuli. They provide detailed information on the topographic distribution, speed, and amplitude of responses and can be acquired using noninvasive, nonconstraining, and portable technology. Researchers have used ERPs to study cortical and thalamocortical activation (Key, Dove, & Maguire, 2005), predict neurodevelopmental outcomes (Maitre, Aschner, Slaughter, & Key, 2013; Molfese et al., 2008), and document effects of behavioral treatments in persons with disabilities (Marchand, D’Arcy, & Connolly, 2002; Molfese, Molfese, Garrod, & Molfese, 2012; Pulvermüller, Hauk, Zohsel, Neininger, & Mohr, 2005).
Cerebral palsy (CP) is the most common pediatric disorder of movement. CP results from sensory and motor impairments due to perinatal brain injury (O’Shea, 2008; Rosenbaum, Paneth, Leviton, Goldstein, & Bax, 2007; Yekutiel, Jariwala, & Stretch, 1994), with lifetime consequences that range from poor adaptive and social function to communication and emotional disturbances (Rosenbaum & Stewart, 2004). Due to abnormalities in thalamocortical and corticocortical pathways, children with CP do not receive accurate sensory feedback from their movements (Auld, Boyd, Moseley, Ware, & Johnston, 2012; Eliasson, Gordon, & Forssberg, 1992), leading to neglect of an affected extremity and difficulty in learning new movements (Houwink, Geerdink, Steenbergen, Geurts, & Aarts, 2013; Taub, Ramey, DeLuca, & Echols, 2004). Lesions to corticospinal and corticothalamic pathways also impair execution of new and complex movements (Hoon et al., 2009).
Constraint-induced movement therapy (CIMT) is routinely used for rehabilitation of adult and pediatric patients with CP (Hoare, Wasiak, Imms, & Carey, 2007; Huang, Fetters, Hale, & McBride, 2009; Sakzewski, Ziviani, & Boyd, 2009). The principle of CIMT is that restraint of the less affected limb, combined with repetition and shaping of movements, improves the function of a paretic arm (Trauner, 2003; Van de Winckel et al., 2013). While structural brain reorganization after rehabilitation in children with hemiparetic CP is insufficiently studied, evidence exists in adults of significant increases in the grey matter of bilateral primary sensory areas and hippocampi after CIMT. In children, increases in sensorimotor and hippocampal grey matter volume (Sterling et al., 2013) have been noted. Functional reorganization is potentially mediated through enhancement of existing neural activity (Hamzei et al., 2006; Staudt, 2010), especially in the cortical and thalamic pathways of the hemisphere ipsilateral to the lesion, or contralateral to the more affected extremity (Inguaggiato, Sgandurra, Perazza, Guzzetta, & Cioni, 2013).
Existing neuroimaging studies have established strong connections between motor and language functions (Boulenger et al., 2006; Dubois et al., 2009; Marino, Gough, Gallese, Riggio, & Buccino, 2013; Pulvermüller, 2005). In particular, language comprehension makes use of functional corticothalamic pathways that are impacted by CIMT (Alitto & Usrey, 2003; Llano, 2013; Wahl et al., 2008). An ERP paradigm based on a passive language comprehension task has been previously used to assess comprehension in a case of a child with CP (Byrne, Dywan, & Connolly, 1995). This paradigm makes of use of the N400 effect, a surface-negative ERP response that, much like a mismatch negativity response to speech sounds, discriminates between expected and unexpected word–picture pairs. The N400 response is of particular interest to the study of CIMT effects because its generators are estimated to be located in bilateral frontal and temporal lobes, the limbic system (Frishkoff, Tucker, Davey, & Scherg, 2004), and the thalamus (Kuperberg et al., 2000). Additionally, the N400 response is well characterized and reflects the influence of lateralized corticothalamic and thalamocortical connections, which are potentially altered after CIMT.
Therefore, we designed a proof-of-concept observational study testing the feasibility of using the Byrne ERP paradigm as an initial approach to the study of neural changes after an intensive camp-based course of CIMT, a rehabilitative model used for children with CP at our institution. Our goal in this pilot study was to determine whether we could document broad changes in patterns of neural activation (ipsilateral and contralateral to the lesion) after CIMT intervention, by using an established ERP paradigm that does not require an overt behavioral response from the participant. We hypothesized that if CIMT had a measurable clinical effect, ERPs would reflect changes in cortical neural processing as well.
METHOD
Study design
This was a prospective observational study of children cared for at Monroe Carrell Jr. Children’s Hospital at Vanderbilt Medical Center who were already participating in a CIMT camp. All behavioral and ERP assessments were performed at three time-points: baseline immediately prior to the intervention (Time 0), immediately post intervention (Time 1), and 6 months post intervention (Time 2). The CIMT model was repeated for two consecutive years.
The Vanderbilt Institutional Review Board (IRB) approved the study protocol. All parents consented, and all children assented to the study using IRB-approved protocols and forms.
Participants
A total of 16 participants were enrolled in the study: 10 in 2010 and 6 in 2011. Inclusion criteria were participation in the CIMT camp, a medical diagnosis of CP, a greater deficit of tone and movement of one upper extremity in comparison to the other as determined by a pediatric neurologist, age 5–12 years, toilet training, and a score of at least 60 on the nonmotor domains of the 60-month Ages and Stages Questionnaire (ASQ), including language (Klamer, Lando, Pinborg, & Greisen, 2005; Yu et al., 2007). Patients with uncontrolled seizure disorder or botulism toxin injection in the affected upper extremity within 6 months of Time 0 were excluded from the study.
The median age of participants was 6 years (range 5–12 years). Girls made up 25% of the study population. The paretic side was the left one in 55% of the children. CP was scored as 1 (80%) and 2 (20%) on the Gross Motor Classification Scale (Palisano, Rosenbaum, Bartlett, & Livingston, 2008). Zancolli wrist classification (Zancolli & Zancolli, 1981) was 1 (60%) and 2A (40%). Etiology of lesions on neuroimaging was verified from the clinical and radio-graphic records and was related to clinical history as follows: prematurity (periventricular leukomalacia/intraventricular hemorrhage) in 70% of participants, neonatal stroke or hemorrhage in 20%, and neonatal mass lesions/lesion removal in 10%. Two families declined to participate in ERP testing. One patient was not a native English speaker and lived in a home where the primary spoken language was Spanish; her 60-month ASQ language scores met inclusion criteria.
Rehabilitative intervention
After Time 0 assessments, a therapist casted the less affected extremity with a nonremovable rigid cast from the proximal humerus to the distal phalanx of the fingers, maintaining the wrist in a neutral position and the hand open (modified from (Deluca, Echols, Law, & Ramey, 2006). At Time 1 (immediately after treatment period), the cast was removed, and suggestions for continued use of the extremity were given to parents. Total cast-wearing time was approximately 120 hours (or 5 full days) per child with 22–25 hours of shaping of movements and repetition by occupational and physical therapists. The daily program consisted of gross motor/bilateral activities focused on balance and proprioception, fine motor activities, and unilateral self-care activities (e.g., food preparation, self-feeding, and washing), together comprising 60% of the therapy. These interventions were considered mixed (motor and sensory) in nature. For the other 40% of therapy time, children participated in reward-driven sensory-based activities focused on temperature, texture, light and deep pressure, and vibration. Examples included searching for “gold-coin treasures” in a container of cold glass beads, mixing and molding clay to make a keepsake, or combing and petting therapy dogs.
Upper limb assessment measures
The Quality of Upper Extremity Skills Test (QUEST) was administered by therapists and measured changes on 36 items in the four domains of dissociative movement, grasp, weight bearing, and protective extension. Choice of this assessment rather than the Assisting Hand Assessment was based on high concurrent validity with the Peabody Developmental Motor Scales (fine motor subscale: 0.84), the items being related to quality of movement, not chronological age (DeMatteo, 1992; Thorley, Lannin, Cusick, Novak, & Boyd, 2012), and the training of the therapists at our institution. Administration required approximately 30–45 minutes, depending on the severity of the child’s paresis. Outcomes measures for the QUEST included four individual domain scores, ranging from 0 to 100. These were analyzed as continuous variables.
The Pediatric Motor Activity Log (PMAL) is a parent-reported measure used to evaluate how well and how often the child used his or her more affected extremity in 22 functional tasks and how much of this use was spontaneous versus prompted by a caregiver. For consistency, the same parent completed the PMAL at all time-points. The revised scale of the PMAL, or PMAL–R (Uswatte et al., 2012), was used, in which the “How Often” and “How Well” scales ranged from 0 to 5 in increments of 0.5. These were analyzed as continuous variables.
ERP assessments
The choice of the Byrne picture–word matching task was motivated by prior studies in toddlers and children, one with CP, as well as the characteristics of the cortical processing involved in this paradigm (Byrne et al., 1995, 1999, 2001; Friedrich & Friederici, 2004). Byrne and colleagues developed a receptive language-based task that does not require active participation: Children are presented with picture–word pairs that are either congruent or incongruent, resulting in different endogenous ERP responses for matched and mismatched pairs. In typical children, the negative peak around 400 ms (N400) is larger for a mismatching than for a matching picture–word pair. The N400 component is sensitive to semantic mismatch regardless of stimulus modality and can be observed in both hemispheres (Key et al., 2005). This paradigm does not require overt movements, thus minimizing any motor-related artifacts. Because our interest was in the overall changes in cortical function following treatment, we chose to focus on activity over the hemispheres ipsi- and contralateral to the lesion, rather than the right–left differences more commonly used in the previous studies of this paradigm.
ERP data acquisition
Data were acquired using a soft 128-channel geodesic sensor net (EGI, Inc., Eugene, OR), with sampling every 4 ms and filters set at 0.1–100 Hz. A subset of prespecified scalp locations was used in the analysis (Figures 1A and B). The electrode clusters were selected to correspond to the typical electrode locations placed according to the 10–20 system (Key et al., 2005). Since our 128-channel array provided higher density of scalp coverage, selecting a cluster of electrodes and averaging their values instead of a single channel was deemed more representative of the observed scalp activity and more reliable. The ERP paradigm was adapted as follows: A total of 46 color drawings of objects from the Peabody Picture Vocabulary Test–IV (PPVT–IV; D. M. Dunn & L. M. Dunn, 2007; L. M. Dunn, 1981) constituted the primes, and 46 spoken words (recorded by a female native English speaker with a region-neutral accent) were the targets.
Figure 1.
Event-related potential (ERP) acquisition in children with hemiparetic cerebral palsy. (A) Electrode distribution in frontal clusters. (B) Child undergoing ERP. F3 indicates frontal left; F4 indicates frontal right.
On each trial, following a 500-ms fixation point, a drawing was presented centrally on a monitor for 1700 ms, followed by a spoken word 700 ms after the picture onset. Intertrial intervals varied randomly between 1400 and 2400 ms to prevent habituation to stimulus onset. Words were presented at 75 dB SPL (A) from two speakers positioned in front of the participant. On 50% of the trials, the words matched the object in the drawing; for the remaining 50% of the trials, the words did not match. Participants were instructed to view the pictures, listen to the words, and decide whether “the computer named the picture correctly.” One half of the stimulus words were chosen to be within the child’s vocabulary based on age norms; more complex words comprised the other 50%, due to a wide variation in academic achievement levels. The task had a total of 92 trials and lasted approximately 8 min.
Duration of the ERP procedure was as follows: Placement of the net, adjustments for electrode contacts and proper positioning of the child in front of the screen took approximately 5–7 min, the ERP paradigm added 8 min, and the removal of the net and towel-drying of the child’s hair took another 5 min, for a total procedure time of 20 min.
Data analysis
Individual ERPs were obtained by segmenting the ongoing EEG on word onset to include a 100-ms prestimulus baseline and a 900-ms poststimulus interval. Trials contaminated by ocular or movement artifacts were rejected from further analysis using an automated screening algorithm in Net Station EEG software v. 4.3 (EGI, Inc., Eugene, OR), followed by a manual review of the automated results. The automated screening criteria were set as follows: For the eye channels, voltage in excess of 140 µV was interpreted as an eye blink, and voltage above 55 µV was considered to reflect eye movements. Any electrode with voltage exceeding 200 µV was considered bad. Individual electrodes with poor signal quality were replaced by reconstructing their data using spherical spline interpolation procedures. If more than 15% of the electrodes within a trial were deemed bad, the entire trial was discarded. For a trial to be included in the remaining analyses, no more than 15 electrodes (12% of the array) could be interpolated. For a participant’s data set to be included in the overall analysis, averages for each stimulus had to be based on a minimum of 10 trials. There were no statistically significant differences between rejection rates across all three time points as well as across conditions, with the final averages based on 24.7 ± 5.3 trials in the match and 25.2 ± 4.5 in the mismatch condition (Supplementary Table 1). The data were then averaged across trials, referenced to an average reference, and baseline-corrected by subtracting the average microvolt value across the 100-ms prestimulus interval from each time point in the poststimulus period. Mean amplitudes were calculated for a preselected subset of the electrodes corresponding to frontal locations in the left and right hemispheres (Figure 1A).
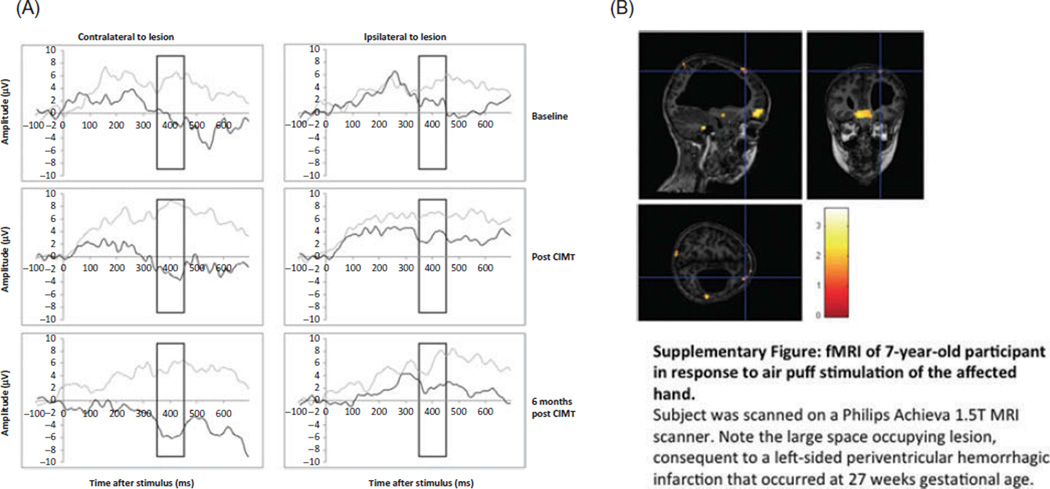
Although N400 response is sometimes described to have a centroparietal maximum (Friedrich & Friederici, 2004), such scalp locations were less optimal for our population due to the large space-occupying lesions underlying these regions, as visualized on neuroimaging in 90% of children (see Supplementary Figure 1). This has been observed in other studies of the N400, in which lesions to temporoparietal areas were linked to a reduced N400 (Friederici, Hahne, & von Cramon, 1998).
The N400 response has been reported at frontal scalp locations in several prior studies (Frishkoff et al., 2004; Key et al., 2005), and selecting them for the present study allowed for more uniform assessment across the study sample. Mean amplitudes were obtained within a 350–450-ms window after stimulus onset, corresponding to the N400 peak previously described.
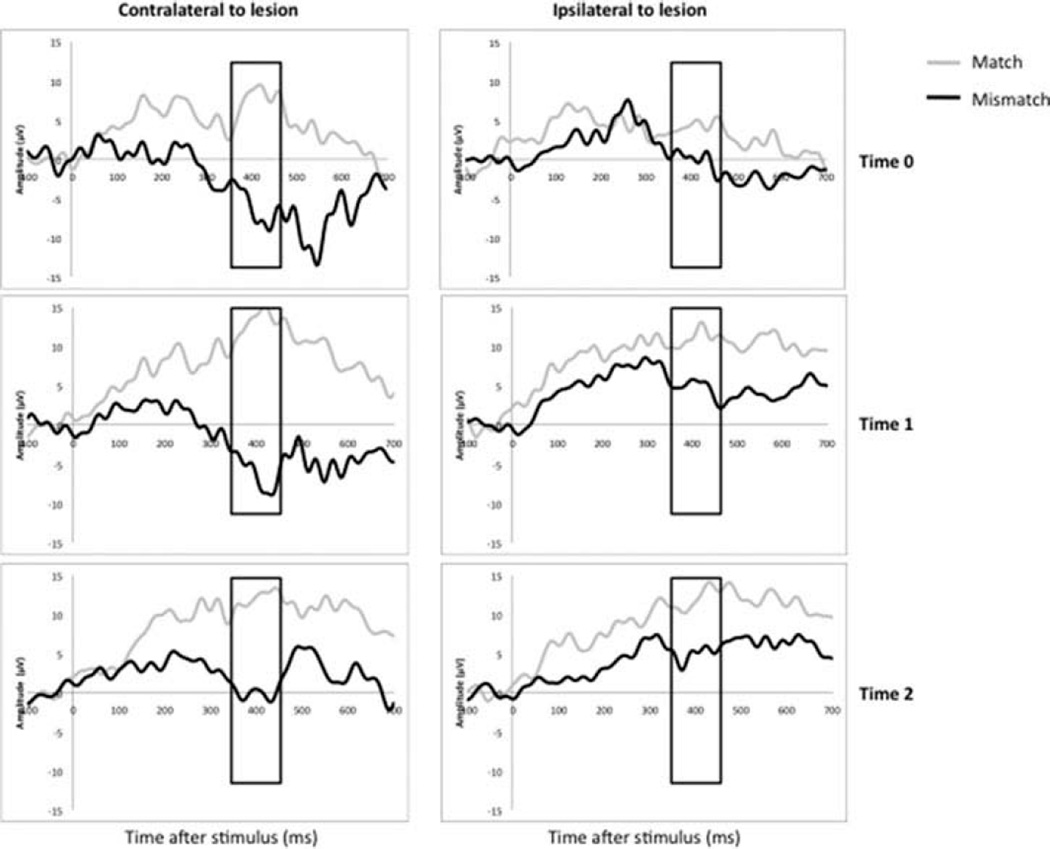
The mismatch and match responses of a representative participant at Times 0, 1, and 2 in hemispheres ipsilateral and contralateral to the lesion are presented in Figure 2. The calculated difference between mean amplitudes in the match and mismatch responses in the selected time window is referred to as the “mismatch-minus-match” (MMM) response. The MMM response was analyzed as a continuous variable.
Figure 2.
Differences in picture–word match and mismatch responses in frontal locations in a representative subject. Response to match is traced in grey, mismatch in black. Panels are vertically arranged by Time 0 (baseline), Time 1 (immediately post CIMT), and Time 2 (6 months post CIMT). Panels are arranged horizontally by hemiscalp locations. Time post stimulus in ms is on the x-axis of each panel, mean amplitude in µV on the y-axis. Rectangle in each panel indicates N400 response window.
To ensure that there were no potential differences in ERPs for more advanced versus known words (as demonstrated by Byrne et al., 1999), we performed repeated measures analysis of variance (ANOVA) with stimulus type as a within-subject factor and demonstrated no significant effects.
Statistical analysis
Continuous variables were summarized using the mean and standard deviation. Categorical variables were summarized using percentages. To evaluate changes within subjects over time, we fit separate linear mixed-effects regression models for each of the PMAL, QUEST, and MMM outcomes. While these models differed in the outcome being studied, they all included two covariates to indicate the timing of the measurement (Time 0, 1, or 2). Because we were interested in within-subject changes in defined outcomes, for which each subject served as his or her own control, no additional adjustment for confounders was considered. Repeated measurements on the same subject over time are likely to be positively correlated; therefore, a random intercept was included in each model to account for this correlation. We determined whether there was an overall change in each outcome due to time using an F-test (two degrees of freedom). Comparisons between Time 0 and Time 1 were made using a Wald test.
Because the ERP paradigm included English words, data from the bilingual (Spanish-speaking) patient were excluded in the final analysis. Data from one patient with a hearing aid were also excluded, as he had not worn his assistive device at Time 1 and Time 2. The final sample in the analysis of ERP data includes 12 patients. To maintain high standards of reproducible research, all analyses were conducted using R statistics software (Version 2.15.1).
RESULTS
Effect of CIMT
All individual measures of the PMAL increased significantly between Time 0 and Time 1, with changes still present at Time 2 (Table 1). The summary PMAL score reflected this trend with an increase from 4.7 (SD = 1.4) at baseline to 7.5 (SD = 1.5) immediately after CIMT and maintenance at 6.6 (SD = 1.5) at 6 months post CIMT, with p < .001 for the trend, as well as the change from Time 0 to Time 1.
TABLE 1.
Effectiveness of CIMT on neurobehavioral measures of arm function: Significance of measurement changes with time
| Test | Time 0a | Time 1a | p Δ0–1b | Time 2a | p Overallc |
|---|---|---|---|---|---|
| PMALd | 4.4 (1.3) | 7.4 (1.7) | <.001 | 6.4 (1.5) | <.001 |
| How Often | 2.1 (0.5) | 3.9 (0.9) | <.001 | 3.1 (0.7) | <.001 |
| How Well | 2.3 (0.8) | 3.5 (0.8) | <.001 | 3.3 (0.8) | <.001 |
| QUESTe | 72 (13) | 78 (11) | .003 | 81 (12) | <.001 |
| Dissociative movement | 64 (22) | 76 (13) | .02 | 80 (14) | .008 |
| Grasp | 67 (18) | 65 (21) | .61 | 76 (21) | .01 |
| Weight bearing | 86 (18) | 91 (8) | .27 | 89 (11) | .52 |
| Protective extension | 71 (21) | 83 (12) | <.001 | 76 (15) | <.001 |
Notes. N = 16. All measures are expressed as mean (standard deviation). PMAL = Pediatric Motor Activity Log; QUEST = Quality of Upper Extremity Skills Test; CIMT = constraint-induced movement therapy.
Time 0: prior to CIMT; Time 1: immediately after CIMT; Time 2: 6 months after CIMT.
p value for Wald test.
p value for F-test.
PMAL summary, How Well and How Often range 0–5.
QUEST summary and domains range 0–100.
Total QUEST, dissociated movement, and protective extension domain scores also showed significant increases immediately after the intervention and at 6 months. The overall increase between all three time points was also significant. Total QUEST increased from 71 (SD = 13) at baseline to 80 (SD = 12) at Time 1 and stayed at 80 (SD = 12) at Time 2 (p < .001), indicating an effective trend in effect of CIMT.
Changes in neural activation relative to lesion side
Participants showed differences in ERP responses to matched versus mismatched picture–word pairs at baseline, similar to the results described in previous studies using this ERP paradigm (Byrne et al., 1995, 1999, 2001; Friedrich & Friederici, 2004). This can be visualized in Figure 2, in the outlined window between 350 and 450 ms surrounding the N400 peak. On the side contralateral to the lesion, the amplitude of the mismatch response appeared larger (more negative) than that of a match and was similar at all three time points. On the side of the brain ipsilateral to the lesion, the difference between match and mismatch appeared less defined at Time 0 but increased by Time 1 and was maintained at Time 2. Averaged tracings for all patients also reflected these changes but had an appearance less typical of ERPs due to heterogeneity of the lesions (see Supplementary Figure 2). These changes were observable not only on ERP tracings but also upon analysis of the quantitative data.
The MMM difference was calculated for each patient in both hemispheres and at all three time-points (Table 2). The MMM value on the side ipsilateral to the lesion increased immediately after the intervention, whereas no such change was noted on the side contralateral to the lesion. Analysis of changes in mean amplitudes between time-points revealed significant increases in the MMM response on the ipsilateral side from –1.2 to –4.2 to –6.3 µV (p = .03). No such trend was observed on the side contralateral to the lesion.
TABLE 2.
Changes in MMM amplitude differences in response to CIMT
| MMM (µV) | Time 0a | Time 1a | p Δ0–1b | Time 2a | p overallc |
|---|---|---|---|---|---|
| Ipsilateral to lesion | −1.2 (3.8) [9.6, −10.2] | −4.2 (6) [5.1, −11.2] | .07 | −6.3 (3.5) [1.4, −14.1] | .03 |
| Contralateral to lesion | −0.54 (4.3) [6.4, −11.2] | −2.1 (3.4) [4.7, −8.3] | .42 | −2.1 (5.4) [5.6, −14.1] | .67 |
Notes. N = 12. All measures are expressed as mean (standard deviation) and [range]. MMM = mismatch-minus-match mean amplitude difference in 350–450-ms time-window after stimulus onset. CIMT = constraint-induced movement therapy.
Time 0: prior to CIMT; Time 1: immediately after CIMT; Time 2: 6 months after CIMT.
p value for Wald test.
p value for F-test.
Associations between ERP measures and functional measures
Change in ipsilateral MMM over the 6-month period (Table 2) showed a trend in correlation with changes in PMAL total score (p = .08), but this relationship appeared driven entirely by the associations between MMM change and PMAL How Well score (p = .02). As the quality of use of the affected extremity improved, MMM became more significantly more negative on the side ipsilateral to the lesion (R = .4, p = .02). For the QUEST, the correlations were moderately large, but with so few subjects, we had inadequate precision to detect a significant association in the data.
DISCUSSION
This pilot study demonstrates the feasibility of using ERP methodology to document functional cortical changes in children with CP who undergo rehabilitative interventions such as CIMT.
Using a brief paradigm and portable set-up, it is possible to rapidly test vulnerable children without requiring active participation, verbalization, or restraint. ERPs were acquired in an off-site facility using standard therapy rooms and offices, underscoring the adaptability of the methodology.
The goal of our study was to test whether ERP could measure neural changes after a routinely used rehabilitative intervention such as CIMT. Therefore we relied on neurobehavioral assessments already used in our clinical setting to document the effect of the intervention, even though they may not be the most precise research tools to measure hand and arm function. For example, the validity of the PMAL and even the PMAL–R has been challenged and narrowed (Chen et al., 2013; Wallen & Ziviani, 2013). However, it remains a valuable tool for documenting the use of the affected extremity in daily activities, especially when combined with other measures of bimanual activities and therapist-administered skills assessments, such as the QUEST. Other measures of upper extremity function, including the Assisting Hand Assessment or Melbourne Assessment of Unilateral Upper Limb Function, could also be used in future research studies (Gordon et al., 2011; Holmefur et al., 2013; Holmefur, Krumlinde-Sundholm, Bergstrom, & Eliasson, 2010; Klingels et al., 2008; Thorley, Lannin, Cusick, Novak, & Boyd, 2011; Thorley et al., 2012).
Combining these assessments with direct neuroimaging can provide more quantitative and direct measures of neural function. The design of the INCITE trial, for example, focuses on using fMRI and TMS to evaluate mechanisms of response to CIMT (Boyd et al., 2010). These methodologies can provide crucial information about rewiring of the brain after CIMT. However, they are difficult to use in young children and toddlers, due to the length (45 min or longer), discomfort, and associated anxiety, as well as requirements for active participation in some cases. Thus, even with the best child-friendly training tools (Hallowell, Stewart, de Amorim E Silva, & Ditchfield, 2008; Slifer, Koontz, & Cataldo, 2002), studies using TMS and fMRI usually involve children aged 7 years or older. Such studies of connectivity and neurostructural changes are essential to understanding the mechanistic bases for neurorehabilitation. Our focus in this pilot was on developing a complementary tool that would allow the study of temporal characteristics of neural activation and processing rather than structure–function relationships and that could be used in younger children.
Our results suggest that ERPs are a useful methodology appropriate for measurements of cortical function in young children, allowing earlier evaluation of intervention effects, during a period of maximal brain plasticity. ERP methodology does not require active participation, is brief (less than 10 min of testing for a paradigm with over 90 trials), and reflects cortical function data with millisecond-level precision (Key et al., 2005). Additionally, ERP equipment is highly portable, allowing it to be adapted to a variety of clinical, educational, and even residential settings, such as a CIMT camp or participants’ homes. The soft sensor nets are well tolerated and not aversive, and the technology preserves both a full visual field and a child’s ability to move. While ERP analysis precludes any definite conclusions regarding specific neural sources of observed activity, it has the potential to be combined with other neuroimaging modalities that can attribute cortical structure relationships (Hari, Parkkonen, & Nangini, 2010).
The increase in ipsilateral cortical activity after CIMT observed in ERP measures in our study concurs with fMRI results from other groups (Juenger et al., 2007), with lasting results at 6 months post CIMT. The reasons for the observed increase in ipsilateral brain activity on fMRI have been attributed to possible remnant activity or integrity of corticospinal tracts (Juenger et al., 2007). However, our ERP paradigm, due to its use of semantic processing and working memory, reflected complex cortical functions beyond basic auditory and visual processing. The N400 is likely to arise from multiple generators that are segregated both functionally (Nobre & McCarthy, 1994) and spatially (Halgren et al., 1994; McCarthy, Nobre, Bentin, & Spencer, 1995). Friederici et al. (1998) observed that lesions to temporoparietal areas may be linked to a reduced N400, and Guillem, N’kaoua, Rougier, and Claverie (1995) recorded intracranial N400s in prefrontal as well as temporal and parietal regions. Results of intracortical recordings also point to the parahippocampal anterior fusiform gyrus (McCarthy et al., 1995; Nobre, Allison, & McCarthy, 1994), medial temporal structures near the hippocampus and amygdala (Halgren et al., 1994; Nobre & McCarthy, 1995; Smith, Stapleton, & Halgren, 1986), and locations in the lateral temporal region (Simos, Basile, & Papanicolaou, 1997). Neuroimaging studies of language have identified significant regions of activation to semantic violations in left inferior frontal cortex (Kiehl, Laurens, & Liddle, 2002; Newman, Twieg, & Carpenter, 2001). Beyond scalp locations, studies of the N400 generators by Frishkoff et al. (2004) have implicated more complex models incorporating association areas, corticothalamic networks involved in attentional engagement, and even corticolimbic regulation of memory access. While we did not attempt source localization in the current pilot study, the enhancement of the N400 in the lesioned hemisphere suggests “top-down” effects of CIMT on cortical reorganization, beyond “bottom-up” sensory pathway changes noted in imaging studies. This “top-down” process would be consistent with regulation of attention to information by the prefrontal cortex to association areas. Increased use of a neglected extremity may enhance attention to sensory stimuli and the ability to evaluate the relevance of sensory inputs versus noise. While this has not been studied in the case of language, asynchrony of tactile processing in sensory-motor cortices contributes to motor performance errors in children with CP (Kurz, Heinrichs-Graham, Arpin, Becker, & Wilson, 2014).
Our study has the limitation of using a higher order language processing measure of cortical activity to assess neural changes due to treatment, thus examining endogenous instead of exogenous responses and purely sensory or perceptual processes (Key et al., 2005). Nevertheless, it provides quantitative evidence for lateralized treatment-related changes in neural activation. Additionally, our small sample size limited in-depth statistical analyses and our ability to derive any mechanistic conclusions, narrowing our focus on the feasibility of ERP.
ERP methodology may prove to be a valuable tool for evidence-based design of pediatric CIMT and other neurorehabilitative therapies. Our future ERP research will test the effects of CIMT directly on cortical sensory processing through the use of somatosensory evoked potentials recorded in a novel paradigm based on light touch, recently developed in our lab, instead of electrical stimulation (Maitre, Barnett, & Key, 2012).
Supplementary Material
Acknowledgments
The project was supported by the National Center for Research Resources [grant number UL1 RR024975-01], now at the National Center for Advancing Translational Sciences [grant number 2 UL1 TR000445-06]; the National Institute of Child Health and Human Development [grant number P30HD15052 to the Vanderbilt Kennedy Center].
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary Table 1 and Figures 1 and 2 are available via the “Supplementary” tab on the article’s online page (http://dx.doi.org/10.1080/13803395.2014.925094).
REFERENCES
- Alitto HJ, Usrey WM. Corticothalamic feedback and sensory processing. Current Opinion in Neurobiology. 2003;13(4):440–445. doi: 10.1016/s0959-4388(03)00096-5. [DOI] [PubMed] [Google Scholar]
- Auld MLM, Boyd RNR, Moseley GLG, Ware RSR, Johnston LML. Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy. Archives of Physical Medicine and Rehabilitation. 2012;93(4):696–702. doi: 10.1016/j.apmr.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Berweck S, Kuhnke N, Walther M, Hadyk T, Landes V, Brodbeck V, Mall V. Constraint-induced movement therapy in congenital hemiparesis with different types of cortico-spinal reorganization: A TMS study. Neuropediatrics. 2006;37(6):FV1. [Google Scholar]
- Boulenger V, Roy AC, Paulignan Y, Deprez V, Jeannerod M, Nazir TA. Cross-talk between language processes and overt motor behavior in the first 200 msec of processing. Journal of Cognitive Neuroscience. 2006;18(10):1607–1615. doi: 10.1162/jocn.2006.18.10.1607. [DOI] [PubMed] [Google Scholar]
- Boyd R, Sakzewski L, Ziviani J, Abbott DF, Badawy R, Gilmore R, Jackson GD. INCITE: A randomised trial comparing constraint induced movement therapy and bimanual training in children with congenital hemiplegia. BMC Neurology. 2010;10:4. doi: 10.1186/1471-2377-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne JM, Connolly JF, MacLean SE, Beattie TL, Dooley JM, Gordon KE. Brain activity and cognitive status in pediatric patients: Development of a clinical assessment protocol. Journal of Child Neurology. 2001;16(5):325–332. doi: 10.1177/088307380101600504. [DOI] [PubMed] [Google Scholar]
- Byrne JM, Connolly JF, MacLean SE, Dooley JM, Gordon KE, Beattie TL. Brain activity and language assessment using event-related potentials: Development of a clinical protocol. Developmental Medicine & Child Neurology. 1999;41(11):740–747. doi: 10.1017/s0012162299001504. [DOI] [PubMed] [Google Scholar]
- Byrne JM, Dywan CA, Connolly JF. An innovative method to assess the receptive vocabulary of children with cerebral palsy using event-related brain potentials. Journal of Clinical and Experimental Neuropsychology. 1995;17(1):9–19. doi: 10.1080/13803399508406576. [DOI] [PubMed] [Google Scholar]
- Chen C-L, Shen I-H, Chen C-Y, Wu C-Y, Liu W-Y, Chung C-Y. Validity, responsiveness, minimal detectable change, and minimal clinically important change of Pediatric Balance Scale in children with cerebral palsy. Research in Developmental Disabilities. 2013;34(3):916–922. doi: 10.1016/j.ridd.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Deluca SC, Echols K, Law CR, Ramey SL. Intensive pediatric constraint-induced therapy for children with cerebral palsy: Randomized, controlled, crossover trial. Journal of Child Neurology. 2006;21(11):931–938. doi: 10.1177/08830738060210110401. [DOI] [PubMed] [Google Scholar]
- DeMatteo C. QUEST: Quality of Upper Extremity Skills Test. Hamilton, ON: McMaster University, Neurodevelopmental Clinical Research Unit; 1992. [Google Scholar]
- Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cerebral Cortex. 2009;19(2):414–423. doi: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- Dunn DM, Dunn LM. Peabody Picture Vocabulary Test. 5th. San Antonio, TX: Pearson; 2007. [Google Scholar]
- Dunn LM. Peabody Picture Vocabulary Test, Revised (Special ed.) Circle Pines, MN: American Guidance Service; 1981. [Google Scholar]
- Eliasson AC, Gordon AM, Forssberg H. Impaired anticipatory control of isometric forces during grasping by children with cerebral palsy. Developmental Medicine & Child Neurology. 1992;34(3):216–225. doi: 10.1111/j.1469-8749.1992.tb14994.x. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Hahne A, von Cramon DY. First-pass versus second-pass parsing processes in a Wernicke’s and a Broca’s aphasic: Electrophysiological evidence for a double dissociation. Brain and Language. 1998;62(3):311–341. doi: 10.1006/brln.1997.1906. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Friederici AD. N400-like semantic incongruity effect in 19-month-olds: Processing known words in picture contexts. Journal of Cognitive Neuroscience. 2004;16(8):1465–1477. doi: 10.1162/0898929042304705. [DOI] [PubMed] [Google Scholar]
- Frishkoff GA, Tucker DM, Davey C, Scherg M. Frontal and posterior sources of event-related potentials in semantic comprehension. Brain Research. Cognitive Brain Research. 2004;20(3):329–354. doi: 10.1016/j.cogbrainres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Hung Y-C, Brandao M, Ferre CL, Kuo H-C, Friel K, Charles JR. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: A randomized trial. Neurorehabilitation and Neural Repair. 2011;25(8):692–702. doi: 10.1177/1545968311402508. [DOI] [PubMed] [Google Scholar]
- Guillem F, N’kaoua B, Rougier A, Claverie B. Intracranial topography of event-related potentials (N400/P600) elicited during a continuous recognition memory task. Psychophysiology. 1995;32(4):382–392. doi: 10.1111/j.1469-8986.1995.tb01221.x. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Chauvel P, Clarke M. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices. Journal of Physiology-Paris. 1994;88(1):51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Hallowell LM, Stewart SE, de Amorim ESilva CT, Ditchfield MR. Reviewing the process of preparing children for MRI. Pediatric Radiology. 2008;38(3):271–279. doi: 10.1007/s00247-007-0704-x. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Liepert J, Dettmers C, Weiller C, Rijntjes M. Two different reorganization patterns after rehabilitative therapy: An exploratory study with fMRI and TMS. NeuroImage. 2006;31(2):710–720. doi: 10.1016/j.neuroimage.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Hari R, Parkkonen L, Nangini C. The brain in time: Insights from neuromagnetic recordings. Annals of the New York Academy of Sciences. 2010;1191:89–109. doi: 10.1111/j.1749-6632.2010.05438.x. [DOI] [PubMed] [Google Scholar]
- Hoare BJ, Wasiak J, Imms C, Carey L. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database of Systematic Reviews. 2007 doi: 10.1002/14651858.CD004149.pub2. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Holmefur M, Kits A, Bergström J, Krumlinde-Sundholm L, Flodmark O, Forssberg H, Eliasson A-C. Neuroradiology can predict the development of hand function in children with unilateral cerebral palsy. Neurorehabilitation and Neural Repair. 2013;27(1):72–78. doi: 10.1177/1545968312446950. [DOI] [PubMed] [Google Scholar]
- Holmefur M, Krumlinde-Sundholm L, Bergstrom J, Eliasson AC. Longitudinal development of hand function in children with unilateral cerebral palsy. Developmental Medicine and Child Neurology. 2010;52(4):352–357. doi: 10.1111/j.1469-8749.2009.03364.x. [DOI] [PubMed] [Google Scholar]
- Hoon AH, Stashinko EE, Nagae LM, Lin DDM, Keller J, Bastian A, Jonston MV. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Developmental Medicine and Child Neurology. 2009;51(9):697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houwink A, Geerdink YA, Steenbergen B, Geurts AC, Aarts P. Assessment of upper-limb capacity, performance, and developmental disregard in children with cerebral palsy: Validity and reliability of the revised Video-Observation Aarts and Aarts module: Determine Developmental Disregard (VOAA-DDD-R) Developmental Medicine and Child Neurology. 2013;55(1):76–82. doi: 10.1111/j.1469-8749.2012.04442.x. [DOI] [PubMed] [Google Scholar]
- Huang H-H, Fetters L, Hale J, McBride A. Bound for success: A systematic review of constraint-induced movement therapy in children with cerebral palsy supports improved arm and hand use. Physical Therapy. 2009;89(11):1126–1141. doi: 10.2522/ptj.20080111. [DOI] [PubMed] [Google Scholar]
- Inguaggiato E, Sgandurra G, Perazza S, Guzzetta A, Cioni G. Brain reorganization following intervention in children with congenital hemiplegia: A systematic review. Neural Plasticity. 2013;2013:356275. doi: 10.1155/2013/356275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juenger H, Linder-Lucht M, Walther M, Berweck S, Mall V, Staudt M. Cortical neuromodula-tion by constraint-induced movement therapy in congenital hemiparesis: An fMRI study. Neuropediatrics. 2007;38(3):130–136. doi: 10.1055/s-2007-985904. [DOI] [PubMed] [Google Scholar]
- Key APF, Dove GO, Maguire MJ. Linking brainwaves to the brain: An ERP primer. Developmental Neuropsychology. 2005;27(2):183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- Kiehl AK, Laurens KR, Liddle PF. Reading anomalous sentences: An event-related fMRI study of semantic processing. NeuroImage. 2002;17(2):842–850. [PubMed] [Google Scholar]
- Klamer A, Lando A, Pinborg A, Greisen G. Ages and Stages Questionnaire used to measure cognitive deficit in children born extremely preterm. Acta Paediatrica. 2005;94(9):1327–1329. doi: 10.1111/j.1651-2227.2005.tb02095.x. [DOI] [PubMed] [Google Scholar]
- Klingels K, De Cock P, Desloovere K, Huenaerts C, Molenaers G, Van Nuland I, Feys H. Comparison of the Melbourne Assessment of Unilateral Upper Limb Function and the Quality of Upper Extremity Skills Test in hemiplegic CP. Developmental Medicine and Child Neurology. 2008;50(12):904–909. doi: 10.1111/j.1469-8749.2008.03123.x. [DOI] [PubMed] [Google Scholar]
- Kuperberg RG, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, Wright IC, Davis AS. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: An fMRI study. Journal of Cognitive Neuroscience. 2000;12(2):321–341. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Heinrichs-Graham E, Arpin DJ, Becker KM, Wilson TM. Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy. Journal of Neurophysiology. 2014;111(1):573–579. doi: 10.1152/jn.00553.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA. Functional imaging of the thalamus in language. Brain and Language. 2013;126(1):62–72. doi: 10.1016/j.bandl.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre NL, Aschner JL, Slaughter JC, Key AP. Cortical speech sound differentiation in the intensive care nursery predicts cognitive and language development in the first 2 years of life. Developmental Medicine & Child Neurology. 2013;55(9):834–839. doi: 10.1111/dmcn.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre NL, Barnett ZP, Key APF. Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy. Journal of Child Neurology. 2012;27(10):1276–1283. doi: 10.1177/0883073811435682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand Y, D’Arcy RCN, Connolly JF. Linking neurophysiological and neuropsychological measures for aphasia assessment. Clinical Neurophysiology. 2002;113(11):1715–1722. doi: 10.1016/s1388-2457(02)00224-9. [DOI] [PubMed] [Google Scholar]
- Marino BFM, Gough PM, Gallese V, Riggio L, Buccino G. How the motor system handles nouns: A behavioral study. Psychological Research. 2013;77(1):64–73. doi: 10.1007/s00426-011-0371-2. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. The Journal of Neuroscience. 1995;15(2):1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese D, Molfese V, Garrod K, Molfese D. Evidence of dynamic changes in brain processing from imaging techniques: Implications for interventions for developmental disabilities. In: Breznitz Z, Rubinsten O, Molfese VJ, Molfese DL, editors. Literacy studies. Vol. 6. Dordrecht: Springer Netherlands; 2012. pp. 5–24. [Google Scholar]
- Molfese DL, Molfese VJ, Beswick J, Jacobi-Vessels J, Molfese PJ, Key AP, Starkey G. Dynamic links between emerging cognitive skills and brain processes. Developmental Neuropsychology. 2008;33(6):682–706. doi: 10.1080/87565640802418647. [DOI] [PubMed] [Google Scholar]
- Newman SD, Twieg DB, Carpenter PA. Baseline conditions and subtractive logic in neuroima-ging. Human Brain Mapping. 2001;14(4):228–235. doi: 10.1002/hbm.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372(6503):260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related ERPs: Scalp distributions and modulation by word type and semantic priming. Journal of Cognitive Neuroscience. 1994;6(3):233–255. doi: 10.1162/jocn.1994.6.3.233. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. The Journal of Neuroscience. 1995;15(2):1090–1098. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea TM. Diagnosis, treatment, and prevention of cerebral palsy. Clinical Obstetrics and Gynecology. 2008;51(4):816–828. doi: 10.1097/GRF.0b013e3181870ba7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Developmental Medicine and Child Neurology. 2008;50(10):744–750. doi: 10.1111/j.1469-8749.2008.03089.x. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F. Brain mechanisms linking language and action. Nature Reviews. Neuroscience. 2005;6(7):576–582. doi: 10.1038/nrn1706. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Hauk O, Zohsel K, Neininger B, Mohr B. Therapy-related reorganization of language in both hemispheres of patients with chronic aphasia. NeuroImage. 2005;28(2):481–489. doi: 10.1016/j.neuroimage.2005.06.038. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M. A report: The definition and classification of cerebral palsy April 2006. Developmental Medicine & Child Neurology. 2007;49:8–14. [PubMed] [Google Scholar]
- Rosenbaum P, Stewart D. The World Health Organization international classification of functioning, disability, and health: A model to guide clinical thinking, practice and research in the field of cerebral palsy. Seminars in Pediatric Neurology. 2004;11(1):5–10. doi: 10.1016/j.spen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Sakzewski L, Ziviani J, Boyd R. Systematic review and meta-analysis of therapeutic management of upper-limb dysfunction in children with congenital hemiplegia. Pediatrics. 2009;123(6):e1111–e1122. doi: 10.1542/peds.2008-3335. [DOI] [PubMed] [Google Scholar]
- Simos PG, Basile LF, Papanicolaou AC. Source localization of the N400 response in a sentence-reading paradigm using evoked magnetic fields and magnetic resonance imaging. Brain Research. 1997;762(1-2):29–39. doi: 10.1016/s0006-8993(97)00349-1. [DOI] [PubMed] [Google Scholar]
- Slifer KJ, Koontz KL, Cataldo MF. Operant-contingency-based preparation of children for functional magnetic resonance imaging. Journal of Applied Behavior Analysis. 2002;35(2):191–194. doi: 10.1901/jaba.2002.35-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Stapleton JM, Halgren E. Human medial temporal lobe potentials evoked in memory and language tasks. Electroencephalography and Clinical Neurophysiology. 1986;63(2):145–159. doi: 10.1016/0013-4694(86)90008-8. [DOI] [PubMed] [Google Scholar]
- Staudt M. Reorganization after pre- and perinatal brain lesions. Journal of Anatomy. 2010;217(4):469–474. doi: 10.1111/j.1469-7580.2010.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krägeloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: A TMS and fMRI study. Brain. 2002;125(10):2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Sterling C, Taub E, Davis D, Rickards T, Gauthier LV, Griffin A, Uswatte G. Structural neuroplastic change after constraint-induced movement therapy in children with cerebral palsy. Pediatrics. 2013;131(5):e1664–e1669. doi: 10.1542/peds.2012-2051. [DOI] [PubMed] [Google Scholar]
- Taub E, Ramey SL, DeLuca S, Echols K. Efficacy of constraint-induced movement therapy for children with cerebral palsy with asymmetric motor impairment. Pediatrics. 2004;113(2):305–312. doi: 10.1542/peds.113.2.305. [DOI] [PubMed] [Google Scholar]
- Thorley M, Lannin N, Cusick A, Novak I, Boyd R. Reliability of the Quality of Upper Extremity Skills Test for children with cerebral palsy aged 2 to 12 years. Physical & Occupational Therapy in Pediatrics. 2011;32(1):4–21. doi: 10.3109/01942638.2011.602389. [DOI] [PubMed] [Google Scholar]
- Thorley M, Lannin N, Cusick A, Novak I, Boyd R. Construct validity of the Quality of Upper Extremity Skills Test for children with cerebral palsy. Developmental Medicine and Child Neurology. 2012;54(11):1037–1043. doi: 10.1111/j.1469-8749.2012.04368.x. [DOI] [PubMed] [Google Scholar]
- Trauner DA. Hemispatial neglect in young children with early unilateral brain damage. Developmental Medicine & Child Neurology. 2003;45(3):160–166. doi: 10.1017/s0012162203000318. [DOI] [PubMed] [Google Scholar]
- Uswatte G, Taub E, Griffin A, Vogtle L, Rowe J, Barman J. The Pediatric Motor Activity Log-Revised: Assessing real-world arm use in children with cerebral palsy. Rehabilitation Psychology. 2012;57(2):149–158. doi: 10.1037/a0028516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Winckel A, Klingels K, Bruyninckx F, Wenderoth N, Peeters R, Sunaert S, Feys H. How does brain activation differ in children with unilateral cerebral palsy compared to typically developing children, during active and passive movements, and tactile stimulation? An fMRI study. Research in Developmental Disabilities. 2013;34(1):183–197. doi: 10.1016/j.ridd.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Wahl M, Marzinzik F, Friederici AD, Hahne A, Kupsch A, Schneider G-H, Klostermann F. The human thalamus processes syntactic and semantic language violations. Neuron. 2008;59(5):695–707. doi: 10.1016/j.neuron.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Wallen M, Ziviani J. Caution regarding the Pediatric Motor Activity Log to measure upper limb intervention outcomes for children with unilateral cerebral palsy. Developmental Medicine and Child Neurology. 2013;55(6):497–498. doi: 10.1111/dmcn.12057. [DOI] [PubMed] [Google Scholar]
- Yekutiel M, Jariwala M, Stretch P. Sensory deficit in the hands of children with cerebral palsy: A new look at assessment and prevalence. Developmental Medicine & Child Neurology. 1994;36(7):619–624. doi: 10.1111/j.1469-8749.1994.tb11899.x. [DOI] [PubMed] [Google Scholar]
- Yu L-M, Hey E, Doyle LW, Farrell B, Spark P, Altman DG The Magpie Trial Follow-Up Study Collaborative Group. Evaluation of the Ages and Stages Questionnaires in identifying children with neurosensory disability in the Magpie Trial follow-up study. Acta Paediatrica. 2007;96(12):1803–1808. doi: 10.1111/j.1651-2227.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- Zancolli EA, Zancolli ERJ. Surgical management of the hemiplegic spastic hand in cerebral palsy. Surgical Clinics of North America. 1981;61(2):395–406. doi: 10.1016/s0039-6109(16)42389-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.