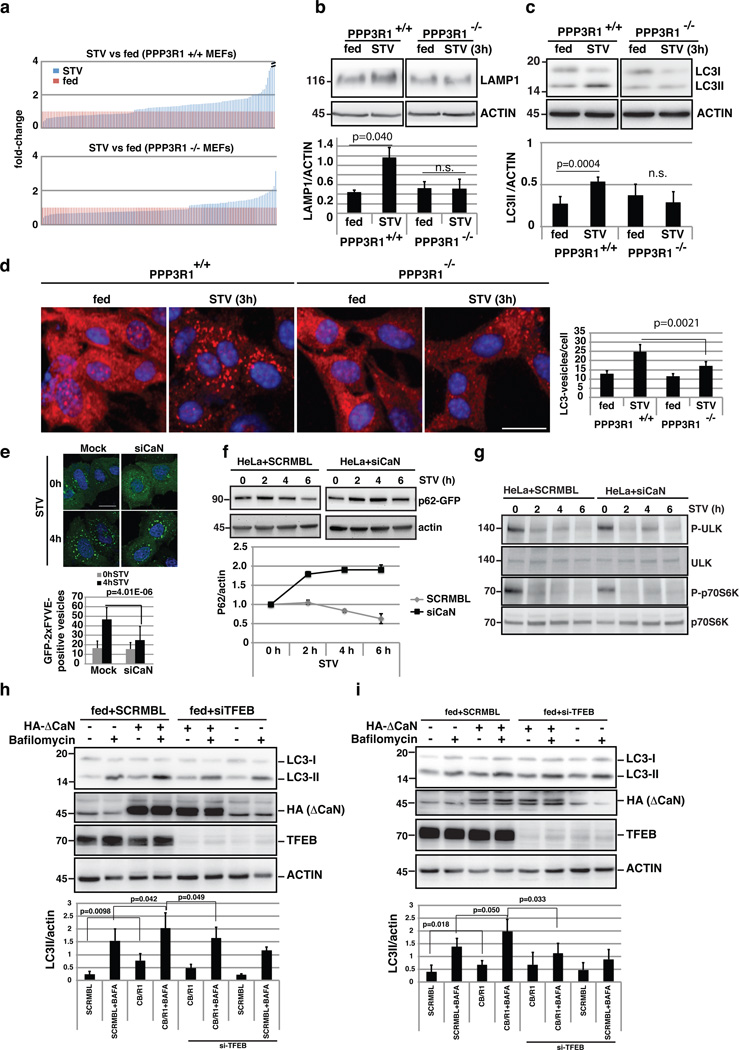
Figure 6.
Calcineurin regulates the lysosomal/autophagic pathway. (a) The transcriptional response of lysosomal/autophagic genes is reduced in PPP3R1−/− KO MEFS (bottom) compared to wild type (top) cells (ranked by fold-change). A value of 1 was assigned to expression levels in fed conditions (n=3 independent experiments). (b-c) Immunoblots against LAMP1 (b), and LC3 (c) from wild type and PPP3R1−/− MEFs in fed and starved (3 hours) conditions. The plot shows the quantification of Lamp1 and LC3-II proteins levels normalized by actin loading control (mean ±s.d for n=2 and n=5 independent experiments, respectively. (d) HC analysis of LC3-positive vesicles in wild type and PPP3R1−/− MEFs in fed and starved conditions. The bar-graph shows the mean ±s.d of LC3-positive vesicles in the different treatment conditions for n= 6 independent experiments. Scale bar 10µm. (e) Analysis of the overexpression of the autophagy-related PI(3)P reporter GFP-2xFYVE55 during starvation in (Mock) HeLa cells, and cells silenced for PPP3CB/PPP3R1. GFP-2xFYVE-positive vesicles were counted using ImageJ software. Approximately 50 cells per condition were analyzed by confocal imaging. Data shows the mean ±s.d for n=2 independent experiments. Scale bar 10µm. (f) Depletion of calcineurin reduces the autophagic flux. HeLa cells were transfected with SCRMBL siRNA or siRNA against both PPP3CB and PPP3R1 (siCaN). After 48 hours, cells were transfected with a plasmid carrying the autophagy substrate p62 fused to GFP for 24h, and treated as indicated. The graph shows the levels of p62-GFP normalized by actin at the different time-points. (g) Analysis of mTOR activity during starvation in normal cells and in cells silenced against calcineurin (siCaN). Two mTOR subtrates, phospho-ULK and phospho-p70S6K, as well as the total protein, were detected by immunoblotting using specific antibodies (n=2 independent experiments). (h,i) Overexpression of ΔCaN increased LC3II protein levels in a TFEB-dependent manner. (h) RPE-1 cells and (i) HeLa were transfected with TFEB or scramble siRNAs and after 48h they were transfected with ΔCaN for 24h. Cells were left in fed conditions and treated or not with bafilomycin. 50µg of protein extracts were then immunoblotted and tested for the amount of LC3-II using specific antibodies (n=4 independent experiments). Uncropped western blots and Source data are provided in Supplementary Figure 8 and Supplementary table 4.