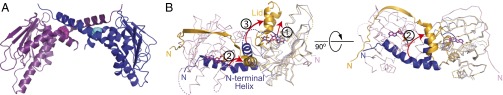
Fig. 2.
Structural comparison of the coiled-coil hTRAP1N dimer (blue/magenta) and the closed-state zTRAP1-ADPNP dimer (gold/pink). (A) Crystal structure of the unliganded hTRAP1N dimer. Phe183 is shown as CPK model. (B) Superposition of unliganded hTRAP1N (blue) onto the structure of the zTRAP1-ADPNP dimer, illustrating the local structural rearrangements in hTRAP1N on nucleotide binding. (1) ATP binding displaces the ATP lid from the nucleotide-binding pocket, concomitant with (2) the N strap undergoing a structural transition from an α-helix to a β-strand that straddles the neighboring subunit and stabilizes the closed-state dimer. (3) Owing to steric interference, the ATP lid must fold over the nucleotide-binding pocket trapping the bound nucleotide.