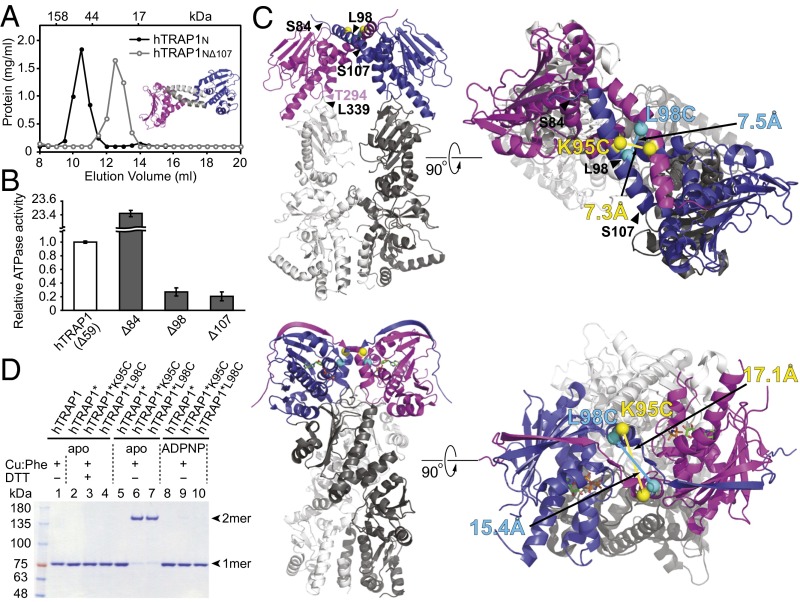
Fig. 3.
Human TRAP1N and hTRAP1 form a coiled-coil dimer. (A) Size-exclusion chromatogram of hTRAP1N (black curve) and hTRAP1NΔ107 (gray curve). (Inset) The hTRAP1N dimer, with the N-terminal helices deleted in hTRAP1NΔ107 shown in gray. (B) Relative ATPase activities of hTRAP1 (Δ59) and N-terminal truncated hTRAP1 mutants lacking the first 84 (Δ84), 98 (Δ98), or 107 (Δ107) residues. (C) In silico model of the intact coiled-coil hTRAP1 dimer (Top) and the crystal structure of the closed-state zTRAP1-ADPNP dimer (21) (Bottom). TRAP1N is shown in blue and magenta; TRAP1MC, in different shades of gray. Introduced Cys sites are marked by spheres. Corresponding Cys pairs are shown in the same color, with distances between Cα atoms indicated. (D) Disulfide cross-linking of hTRAP1 and Cys-containing hTRAP1* variants without or with ADPNP. Cross-linked dimers can be monomerized with 100 mM DTT (+DTT). Bands corresponding to hTRAP1 monomers (1mer) and dimers (2mer) are indicated.