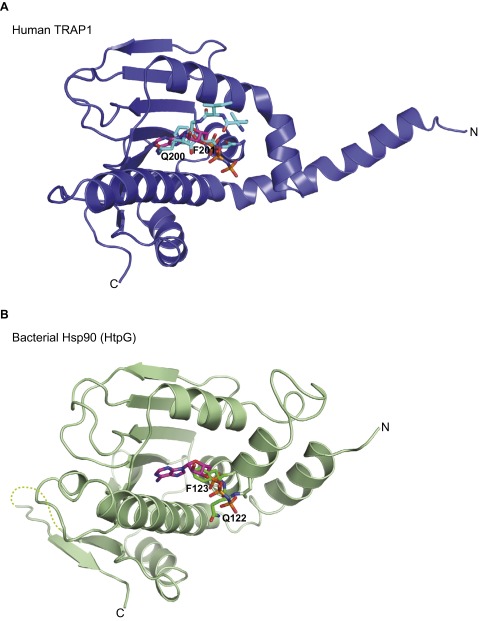
Fig. S8.
The ATP lid of Hsp90 chaperones binds to the ATP-binding pocket in the absence of nucleotide. ADPNP and conserved Gln and Phe residues of the ATP-lid G2 motif in mitochondrial (TRAP1) and bacterial Hsp90 (HtpG) are shown as stick models. Only ADPNP of the nucleotide-bound complex is shown. (A) Superposition of hTRAP1N and hTRAP1N-ADPNP. (B) Superposition of full-length bacterial Hsp90 (PDB ID code 2IOQ) (18) and hTRAP1N-ADPNP. Only the N domain of bacterial Hsp90 is shown.