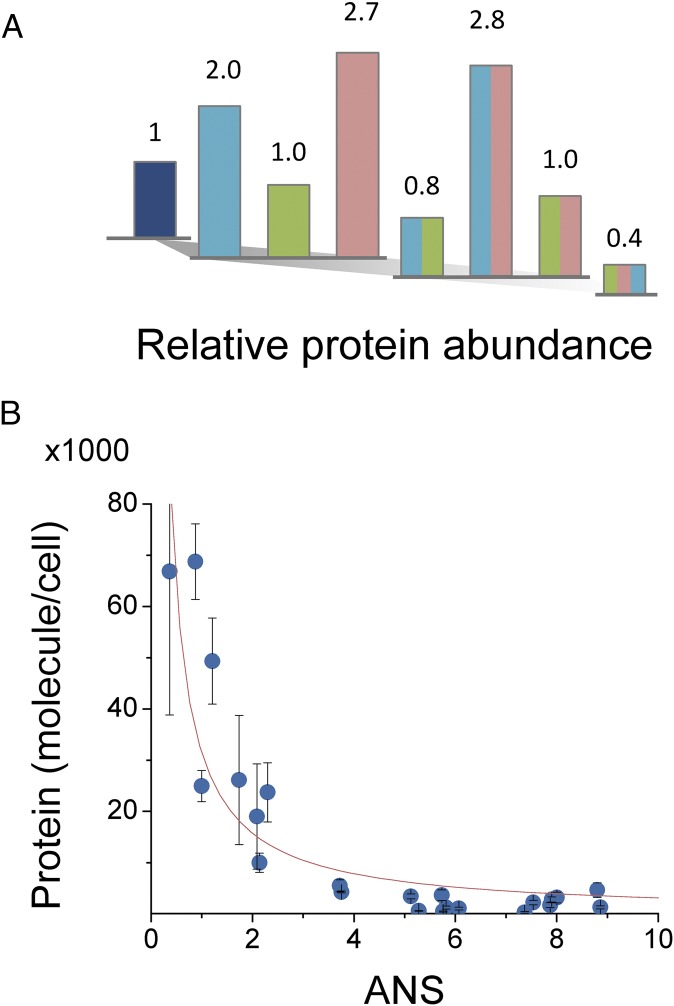
Fig. 2.
Mutations associated with TMP resistance affect the intracellular abundance of DHFR. (A) Intracellular abundance of E. coli DHFR mutants expressed from pFLAG plasmid in the absence of inducer (color scheme as in Fig. 1B). Abundance was determined from total catalytic activity measurements in cell lysates prepared from cultures of different mutants grown at 37 °C in M9 minimal media. Values are normalized to WT DHFR expressed from plasmid at the same conditions. (B) Protein abundance is inversely correlated with propensity to form molten-globule intermediates, as assessed by bis-ANS fluorescence. The represented data (mean ± SEM) were obtained from abundance measurements of E. coli DHFR mutants and orthologous DHFRs from L. grayi and C. muridarum including the cognate TMP-resistance mutations. The fit that is shown was obtained using the equation y = A/(γ·ANS), where A = 4.7 × 105 molecules/cell and γ = 1.5.