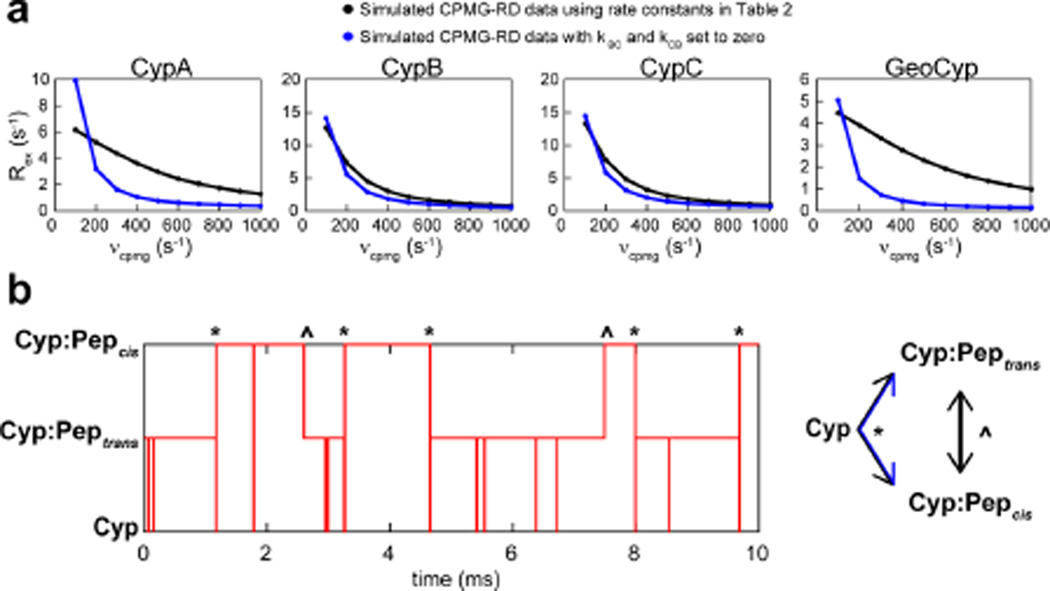
Figure 6.
CPMG-RD can report on uncatalyzed exchange in reversible enzymatic systems. (a) Using the rate constants determined from line shape analysis (Table 2), CPMG-RD data were simulated from the cyclophilin perspective with (black) or without (blue) on-enzyme catalysis, with in silico concentrations of 1 mM cyclophilin and 10 mM peptide. For all cyclophilins, significant exchange persists even in the absence of catalysis. (b) State map of a single simulated atom over a 10 ms simulation, using the microscopic rate constants determined for CypC. Vertical lines represent transitions between states, while horizontal lines represent time spent in a single state. Transitions between Cyp:Peptrans and Cyp:Pepcis occur both through on-enzyme catalysis (^) and through the free enzyme intermediate (*).