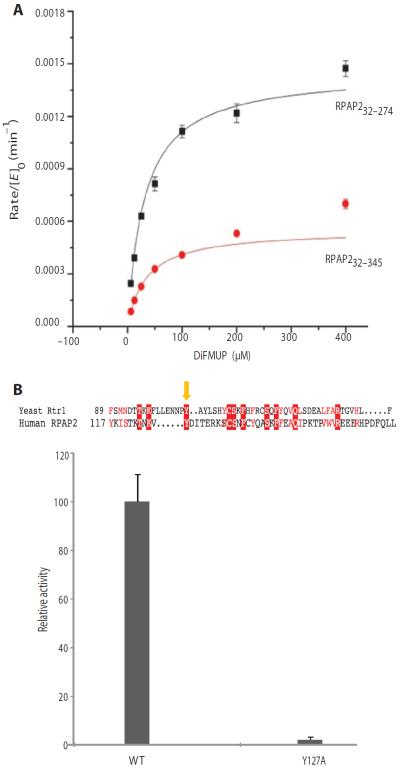
Fig. 8. Phosphoryl transfer activity of human RPAP2 in the DiFMUP assay.
(A) Kinetic parameters of phosphatase activity of RPAP232–274 and RPAP232–345. (B) Tyr105 function is conserved for phosphoryl transfer reaction of Rtr1 and RPAP2. The insert shows the sequence alignment of yeast (S. cerevisiae) Rtr1 and RPAP2, with the yellow arrow indicating the conserved tyrosine at the 105 position of Rrt1 and 127 in RPAP2. Effect of Y127A mutation on the phosphatase activity of RPAP2 in DiFMUP assay is shown at the bottom. The error bars represent SD from triplicate samples, and data are representative of three independent experiments.