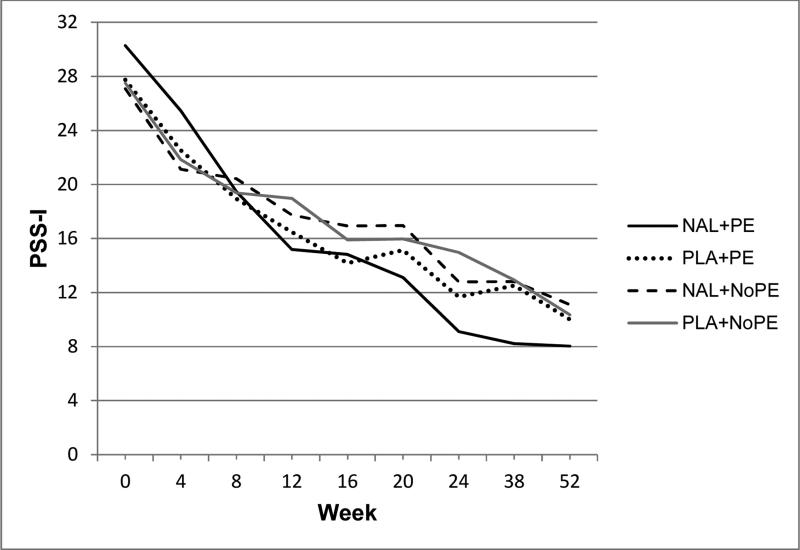
Figure 1.
Raw mean PSS-I (PTSD Symptom Severity Interview) scores during treatment (weeks 1-24) and follow-up (weeks 38 and 52). Treatment conditions: naltrexone with PE (NAL+PE), placebo with PE (PLA+PE), naltrexone with no PE (NAL+NoPE), and placebo with no PE (PLA+NoPE). All participants also received BRENDA (supportive counseling).