Abstract
Introduction
Post-prandial hyperglycemia (PPH) among people with diabetes is a well-known clinical challenge to diabetes management. While the economic burden of diabetes is well studied, little is known about economic costs specific to PPH. The purpose of this study was to investigate costs of PPH related to work, diabetes management, and use of healthcare resources among people with diabetes taking bolus insulin.
Methods
Data were collected in a web survey of 906 adults with type 1 (39%) and type 2 (61%) diabetes taking bolus insulin in Germany (34%), the UK (26%), and the USA (40%).
Results
Sixty-two percent of respondents experienced PPH in the past week, and respondents averaged 1.7 episodes per week. Working respondents indicated that PPH affected their work productivity: 27% missed work time and 71% experienced work productivity issues while at work due to a recent episode of PPH. In terms of diabetes management, respondents with PPH in the past week measured their blood glucose (BG) more frequently than those without PPH (3.7 vs. 2.5 times/day, P < 0.001). PPH was also significantly associated with greater use of healthcare resources. Compared to those without PPH, respondents with PPH reported greater contact with healthcare professionals related to diabetes in the past year (5.5 vs. 4.4 visits, P < 0.001; 2.7 vs. 1.4 calls/emails, P < 0.001) and were more likely to report medical complications related to diabetes (72% vs. 55%, P < 0.001). Average annual costs associated with PPH due to missed work time, additional BG test strips, and physician visits were estimated to be $1239 USD per employed person in the USA.
Conclusion
Results indicate that PPH is associated with greater economic costs and that reducing the incidence of PPH would help mitigate such costs. Additional research is needed to better understand costs associated with PPH that may be more difficult to measure, as well as more long-term impacts and costs.
Funding
Novo Nordisk.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-016-0154-2) contains supplementary material, which is available to authorized users.
Keywords: Diabetes management, Diabetes mellitus, Economic costs, Healthcare resources, Post-prandial hyperglycemia, Work productivity
Introduction
It is well established that better glycemic control among people with diabetes, as indicated by HbA1c values, is associated with a reduced risk of microvascular complications related to diabetes, and there is evidence that glycemic control reduces the risk of macrovascular complications in type 1 diabetes mellitus (T1DM) and in type 2 diabetes mellitus (T2DM) when control is achieved early in the course of the disease [1–4]. While research and diabetes management practices have long focused on HbA1c and fasting plasma glucose as indicators of glycemic control, more recent research highlights the importance of considering post-prandial blood glucose (PPG) as well for a more complete understanding of glycemic control [5]. There is now growing evidence that post-prandial hyperglycemia (PPH) may be particularly important for improving diabetes control, thereby reducing the risk of diabetes-related complications [2, 6]. Further, PPG control is particularly challenging for people with diabetes who take bolus insulin and must calculate and time their dose, which requires coordination with their daily scheduling, as well as remembering to take doses with them [7].
Recent research suggests that PPH has a negative impact on a number of health and daily functioning outcomes in people with diabetes [2]. There is evidence that PPH (or elevated post-challenge glucose following an oral glucose tolerance test) is associated with an increased incidence of cardiovascular disease (CVD) and cardiovascular events [8–12]. PPH is also associated with reduced cognitive functioning among aged people with T2DM [13]. Further, research suggests that PPH (or elevated post-challenge glucose) increases the risk of pancreatic cancer mortality, CVD mortality, and all-cause mortality [9, 14–18].
Given the negative health and functioning impacts associated with PPH among people with diabetes, there is also likely to be an economic burden related to PPH. While the economic cost of diabetes care and treatment is a well-known burden to healthcare budgets [19], little is known about the specific costs associated with PPH. The purpose of this study is to investigate the burden of PPH among people with T1DM and T2DM and related short-term costs associated with PPH in the areas of diabetes management, use of healthcare resources, and work.
Methods
A web survey of people diagnosed with T1DM or T2DM and treated with self-administered basal plus bolus insulin was conducted in Germany, the UK, and the USA. Prior to commencement, the study received ethics approval from Copernicus Group IRB (# TBG1-11-116).
Survey Development
The process of survey development, including conducting focus groups and cognitive debriefing of the survey, followed Food and Drug Administration (FDA) guidelines for development of patient-reported outcomes (PROs) and accepted methodology for concept elicitation to ensure face validity and that items are comprehensive, relevant, and understandable to respondents [20]. As the survey collected data on the patient perspective, as per FDA guidelines, patients were considered the gold standard in generating item content. Nine semi-structured focus groups with a total of 77 people diagnosed with T1DM or T2DM and taking bolus insulin were conducted in Germany (n = 20), the UK (n = 17), and the USA (n = 40) to inform survey development. Transcript data from the focus groups were analyzed using adapted grounded theory [21], and thematic saturation occurred by the eighth focus group. Following survey development, survey items were assessed using cognitive debriefing with 12 individuals diagnosed with diabetes to ensure that survey instructions and items were clear, relevant, easy to understand, and inoffensive. Additionally, the cognitive debriefing was used to ensure that survey structure and recall periods were appropriate. Respondents were given the survey in the local language, and a professional translator was used to ensure consistency. The final survey was composed of 85 questions and took respondents approximately 30 min to complete. Respondents were given modest honoraria (approximately $15 USD per respondent) for completing the survey.
Participants
The web survey was administered to panels of respondents in Germany, the UK, and the USA between July and November of 2013. Inclusion/exclusion criteria required that participants be adults aged 18 years and older who were diagnosed with T1DM or T2DM by a physician or healthcare professional, and treated with bolus insulin therapy but not using pre-mixed insulin or glucagon-like peptide-1 analogs, with or without oral anti-diabetic drug (OAD) use, were eligible to complete the survey. Approximately, 7% of people approached for the survey were eligible to complete the survey based on the inclusion and exclusion criteria. Recruitment quotas were also used for country, age, work status, diabetes type, and method of insulin administration to achieve adequate sampling across various groups and to ensure a representative sample.
Survey Variables
All survey items were self-reported by respondents, and survey items included demographic and diabetes-related characteristics, experience of PPH, missed work time and reduced work productivity related to PPH, blood glucose (BG) measurement, and contact with physicians and other healthcare professionals. Subject characteristic survey measures included age, gender, ethnicity, marital status (percent married/partnered), level of education (percent with college degree), whether or not they work for pay, and the average number of hours worked per week. Diabetes and general health measures included age of diabetes diagnosis, age first took insulin, insulin method (needle/syringe, prefilled pen/durable pen, or insulin pump), how well diabetes controlled (percent indicating well/very well controlled as opposed to moderately/poorly/very poorly controlled), and general health status (percent indicating good/very good/excellent health as opposed to fair/poor health).
Experience of out-of-range PPG (BG after eating) was respondent-reported and based on the survey question, “In the last week (7 days), how many times did you have high/low blood sugar after eating?” PPG measures included whether respondent experienced PPH or post-prandial hypoglycemia in the past week, and the number of times respondent experienced PPH or post-prandial hypoglycemia in the past week. A 5% trim was conducted at the top levels to exclude possible reporting errors to calculate mean number of episodes. Respondents also reported on whether their most recent incident of out-of-range PPG was PPH or post-prandial hypoglycemia (or unsure), and the number of days since their most recent incident of out-of-range PPG (either PPH or post-prandial hypoglycemia).
Missed work time and reduced work productivity due to PPH were restricted to those whose most recent incident of out-of-range PPG was PPH (rather than post-prandial hypoglycemia or being unsure). Respondents indicated missed work time due to PPH (went into work late, left work early, missed a full day of work), and total missed work time (measured in minutes) due to PPG being out-of-range in the past week. The survey also measured presenteeism [22], defined as experience of reduced work productivity/functioning due to PPH (including missed/rescheduled meetings or appointments, making more mistakes, needing to take a break, difficulty focusing, and being less productive). Both missed work and presenteeism were respondent-reported as specifically due to PPH.
BG measurement variables included the number of times respondent measures BG on an average day, and in general, the number of additional times respondent measures BG when experiencing symptoms of hyperglycemia compared to a normal day. Healthcare resource utilization variables included the number of visits to a physician/healthcare professional related to diabetes in the past year, the number of calls/emails to physician/healthcare professional related to diabetes in the past year, and whether or not respondent has been diagnosed with any medical complications as a result of diabetes, including eye problems, nerve damage, cardiovascular problems and/or disease, kidney (renal) disease, high blood pressure, amputations, or other medical problems.
Costs associated with PPH were calculated based on missed work time, increased use of BG monitoring strips, and increased healthcare utilization. Estimates for the productivity costs of missed work time were calculated using respondent income. Employed respondents reported either hourly income or annual income. For those who indicated annual income, hourly income was computed by multiplying respondent-reported work hours per week times estimated work weeks per year for each country (Germany = 40, UK = 46, USA = 47, based on data from the Organisation for Economic Co-operation and Development [23] and the US Bureau of Labor Statistics [24]) to get an approximate total number of work hours per year and then dividing reported annual income by the estimated total number of work hours per year. Income was converted to USD based on exchange rates on July 22, 2013 (1 GBP = 1.5370 USD; 1 Euro = 1.3184 USD). A 5% trim at the top level was used for hourly income to exclude probable reporting errors. For respondents who did not wish to report income, average hourly income was used ($19.26 USD/h). Respondent missed work hours in past week was then multiplied by respondent hourly income to obtain an estimated cost of missed work time due to BG being out-of-range (either high or low BG) after eating in the past week. This estimate was then multiplied by the estimated average number of weeks worked per year for each country (Germany = 40, UK = 46, USA = 47) to get an approximate estimate of the productivity costs of missed work time associated with PPH per year.
To estimate the approximate cost of increased BG monitoring associated with PPH, the difference between those with and without PPH in the past week in the mean number of times respondents measure BG on an average day was calculated. This estimated additional BG tests associated with PPH per person per day was then multiplied by 365 (number of days in a year) to estimate an approximate number of additional BG tests associated with PPH per person annually. The estimated number of additional BG tests per person annually was then multiplied by the average cost of a BG test strip in each country to provide conservative estimates of the average annual costs of additional BG measurement due to PPH for each country. An average cost of 0.20 € ($0.26 USD) per BG test strip was used for Germany [25], $0.98 USD per BG test strip for the USA [26], and £0.24 GBP ($0.37 USD) per BG test strip for the UK [27], using the currency exchange rates noted above to calculate the USD equivalents.
The costs of additional physician/healthcare professional office visits significantly associated with PPH were estimated based on respondents’ reported number of physician/healthcare professional office visits related to diabetes per year. The average number of additional annual office visits for those who experienced PPH compared to those without PPH was multiplied by the average cost of a physician/healthcare office visit for diabetes care in each country to estimate the annual costs of physician/healthcare office visits per year associated with PPH. Conservative estimates were used for physician/healthcare professional office visit costs. For the UK, an average cost of £105.00 ($161.39 USD, using currency exchange rate noted above) per visit was used, based on the average cost of non-consultant led outpatient diabetes care visits, which is less expensive than the average cost for consultant led outpatient visits [28]. For the USA, an average cost of $147.00 USD per visit was used for the USA, based on the average cost of outpatient physician office visits for general diabetes care (excluding visits for diabetes-related complications, which are generally more expensive, on average) [19]. Due to non-significant results, costs of additional physician visits associated with PPH were not estimated for Germany.
Data Analysis
All data were analyzed using SPSS Statistics Software, version 22 (IBM Corporation, Armonk, NY, USA). Analyses included descriptive statistics (means, standard deviations, ranges, and frequency percentages) and measures of association (comparison of means and cross-tabulations). Significance tests were also conducted. For comparison of means between two groups, t tests were used, and for comparison of means among three or more groups, analysis of variance was used [29]. The Chi-square test statistic was used to determine the significance of associations between categorical variables. All analyses were conducted by country and by diabetes type. Among people with T2DM, analyses revealed that results were generally similar whether or not respondents were taking OADs, so these results are not presented here. Comparisons were not possible for people with T1DM, as the vast majority were not taking OADs.
Results
Sample Descriptive Statistics
A total of 906 respondents completed the survey [39% T1DM (n = 356); 61% T2DM (n = 550)]. As expected, respondents with T1DM were significantly younger on average compared to those with T2DM (37.4 vs. 47.4 years, respectively, P < 0.001; Table 1). Respondents with T1DM also had a significantly younger mean age of diagnosis compared to those with T2DM (20.2 vs. 35.6 years, P < 0.001), as well as a significantly younger average age first took insulin (20.9 vs. 39.6 years, P < 0.001). Respondents with T1DM were significantly more likely than those with T2DM to indicate being in “good,” “very good,” or “excellent” health (69.1% vs. 49.6%, P < 0.001). Respondents with T1DM were significantly more likely than those with T2DM to indicate that their diabetes was “well” or “very well” controlled (66.3% vs. 49.6%, P < 0.001). The full sample descriptive statistics by country are presented in Table 1.
Table 1.
Descriptive statistics and diabetes/health characteristics by country
| Characteristics | Germany (n = 305) | UK (n = 236) | USA (n = 365) | Total (n = 906) | |
|---|---|---|---|---|---|
| Age | T1DM, mean(SD)*** | 32.0 (12.0) | 40.1 (13.6) | 41.0 (15.9) | 37.4 (14.4) |
| T2DM, mean(SD)*** | 43.7 (16.2) | 44.5 (15.4) | 51.2 (15.7) | 47.4 (16.2) | |
| Gender*** | n (%) male | 226 (74.1) | 127 (53.8) | 159 (43.6) | 512 (56.5) |
| White/Caucasian*** | n (%) | 297 (97.4) | 216 (91.5) | 302 (82.7) | 815 (90.0) |
| Marital status | n (%) married/partnered | 184 (60.3) | 148 (62.7) | 202 (55.3) | 534 (58.9) |
| Education*** | n (%) college degree | 81 (26.6) | 132 (55.9) | 146 (40.0) | 359 (39.6) |
| Employed*** | n (%) yes | 202 (66.2) | 155 (65.7) | 162 (44.4) | 519 (57.3) |
| Work hours per week** | Mean (SD) | 37.0 (9.5) | 32.6 (13.8) | 35.4 (11.7) | 35.2 (11.7) |
| Diabetes/health characteristics | |||||
| Age diagnosed | T1DM, mean (SD) | 19.8 (11.3) | 19.5 (11.7) | 21.4 (13.2) | 20.2 (12.1) |
| T2DM, mean (SD)*** | 34.0 (13.8) | 32.9 (13.9) | 38.1 (13.8) | 35.6 (14.0) | |
| Diabetes type*** | n (%) T1DM | 130 (42.6) | 117 (49.6) | 109 (29.9) | 356 (39.3) |
| n (%) T2DM | 175 (57.4) | 119 (50.4) | 256 (70.1) | 550 (60.7) | |
| Age 1st took insulin | T1DM, mean (SD) | 20.4 (11.6) | 20.2 (12.0) | 22.3 (13.8) | 20.9 (12.4) |
| T2DM, mean (SD)*** | 36.6 (15.7) | 36.5 (15.5) | 43.1 (15.7) | 39.6 (16.0) | |
| Insulin method*** | n (%) syringe | 83 (27.2) | 51 (21.6) | 164 (44.9) | 298 (32.9) |
| n (%) prefilled pen | 157 (51.5) | 170 (72.0) | 160 (43.8) | 487 (53.8) | |
| n (%) insulin pump | 65 (21.3) | 15 (6.4) | 41 (11.2) | 121 (13.4) | |
| How well diabetes controlled*** | n (%) well/very well | 216 (70.8) | 127 (53.8) | 166 (45.5) | 509 (56.2) |
| Health status* | n (%) good/very good/excellent | 163 (53.4) | 153 (64.8) | 203 (55.6) | 519 (57.3) |
| Diet and exercise habits | |||||
| Number of skipped meals in past week | Mean(SD) | 1.5 (2.5) | 1.3 (1.8) | 1.2 (2.0) | 1.4 (2.1) |
| Ate at restaurant in past week** | n (%) yes | 154 (50.5) | 109 (46.2) | 214 (58.6) | 477 (52.6) |
| Ate takeout in past week* | n (%) yes | 100 (32.8) | 78 (33.1) | 152 (41.6) | 330 (36.4) |
| Ate at friend’s home in past week*** | n (%) yes | 170 (55.7) | 82 (34.7) | 135 (37.0) | 387 (42.7) |
| Light physical activity past week** | Mean hours (SD) | 8.3 (7.2) | 6.5 (7.2) | 6.6 (7.8) | 7.2 (7.5) |
| Moderate physical activity past week** | Mean hours (SD) | 4.3 (4.8) | 2.8 (4.1) | 3.2 (6.1) | 3.5 (5.2) |
| Hard physical activity past week** | Mean hours (SD) | 2.0 (3.5) | 1.5 (3.3) | 1.1 (2.3) | 1.5 (3.0) |
| Physical activity at worka | n (%) yes | 40 (19.8) | 33 (21.3) | 35 (21.6) | 108 (20.8) |
Chi-square/analysis of variance tests indicate significant differences by country: * P < 0.05; ** P < 0.01; *** P < 0.001
SD standard deviation, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus
aThe question about physical activity at work was only asked of employed respondents
Respondent Experiences of PPH
PPH was a frequent occurrence among respondents; 61.9% of respondents reported experiencing PPH in the past week, and 30.0% experienced three or more episodes of PPH in the past week (Table 2). On average, respondents experienced 1.7 episodes of PPH in that past week. In contrast, post-prandial hypoglycemia was relatively less frequent with 35.8% of respondents experiencing post-prandial hypoglycemia in the past week and 11.3% experiencing 3 or more episodes of post-prandial hypoglycemia. On average, respondents reported 0.6 episodes of post-prandial hypoglycemia in that past week. The average number of days since respondents’ last incident of out-of-range post-prandial BG (either hyperglycemia or hypoglycemia) was 7.5 days, suggesting that many who have such events experience them frequently.
Table 2.
Recent experience of PPH and post-prandial hypoglycemia by diabetes type
| T1DM (n = 356) | T2DM (n = 550) | Total (n = 906) | ||
|---|---|---|---|---|
| Experienced PPH in past week | n (%) yes | 219 (61.5) | 342 (62.2) | 561 (61.9) |
| 1 episode | n (%) yes | 46 (12.9) | 80 (14.5) | 126 (13.9) |
| 2 episodes | n (%) yes | 70 (19.7) | 93 (16.9) | 163 (18.0) |
| 3 or more episodes | n (%) yes | 103 (28.9) | 169 (30.7) | 272 (30.0) |
| Number of times experienced PPH in past weeka | Mean (SD) | 1.6 (1.8) | 1.8 (2.1) | 1.7 (1.9) |
| Experienced post-prandial hypoglycemia in past week*** | n (%) yes | 153 (43.0) | 171 (31.1) | 324 (35.8) |
| 1 episode | n (%) yes | 58 (16.3) | 83 (15.1) | 141 (15.6) |
| 2 episodes | n (%) yes | 41 (11.5) | 40 (7.3) | 81 (8.9) |
| 3 or more episodes | n (%) yes | 54 (15.2) | 48 (8.7) | 102 (11.3) |
| Number of times experienced post-prandial hypoglycemia in past weekb,*** | Mean (SD) | 0.8 (1.1) | 0.5 (0.9) | 0.6 (1.0) |
| Number of days since last incident of post-prandial blood glucose instability | Mean (SD) | 8.1 (13.9) | 7.1 (13.1) | 7.5 (13.4) |
Chi-square/t tests indicate significant differences by diabetes type: *** P < 0.001
PPH post-prandial hyperglycemia, SD standard deviation, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus
aData were trimmed by 5% at top level to exclude probable reporting errors (n = 859)
bData were trimmed by 5% at top level to exclude probable reporting errors (n = 867)
Respondents in the USA were significantly more likely to report experiencing PPH in the past week (66.6%) compared to those in Germany (63.0%) and the UK (53.4%, P < 0.01). Additionally, respondents in the USA reported a significantly greater average number of incidents of PPH in the past week (2.0), compared to respondents in the UK (1.5) and Germany (1.5, P < 0.01). Experience of PPH did not differ significantly by diabetes type.
PPH and Missed Work Time and Work Productivity Issues
Among working respondents whose most recent episode of out-of-range PPG was PPH (as opposed to post-prandial hypoglycemia or being unsure), 27.0% reported any missed work time due to that episode of PPH (Table 3). More specifically, 13.7% of respondents indicated that they went in late to work due to PPH, 18.6% indicated that they left early, and 9.9% reported missing a full day of work. On average, respondents reported a total of 168.2 min of missed work time in the past week due to BG being out-of-range after eating (either PPH or post-prandial hypoglycemia). There were no significant differences in reported missed work time by diabetes type or by country.
Table 3.
Missed work time and work productivity issues due to post-prandial hyperglycemia among employed respondents by diabetes type
| T1DM (n = 115) | T2DM (n = 148) | Total (n = 263) | ||
|---|---|---|---|---|
| Any missed work time in past week | n (%) yes | 26 (22.6) | 45 (30.4) | 71 (27.0) |
| Went in late to work | n (%) yes | 13 (11.3) | 23 (15.5) | 36 (13.7) |
| Left work early | n (%) yes | 18 (15.7) | 31 (20.9) | 49 (18.6) |
| Missed a full day of work | n (%) yes | 12 (10.4) | 14 (9.5) | 26 (9.9) |
| Total missed work time in past week due to BG being out-of-range after eating (minutes)a | Mean (SD) | 150.4 (186.9) | 178.5 (181.3) | 168.2 (182.6) |
| Any work productivity issues* | n (%) yes | 72 (62.6) | 114 (77.0) | 186 (70.7) |
| Missed work meetings/appointments | n (%) yes | 9 (7.8) | 16 (10.8) | 25 (9.5) |
| Canceled/rescheduled a meeting/appointment | n (%) yes | 11 (9.6) | 18 (12.2) | 29 (11.0) |
| Made more mistakes at work | n (%) yes | 25 (21.7) | 48 (32.4) | 73 (27.8) |
| Needed to take a break at work | n (%) yes | 46 (40.0) | 69 (46.6) | 115 (43.7) |
| Found it difficult to focus** | n (%) yes | 50 (43.5) | 93 (62.8) | 143 (54.4) |
| Was less productive at work* | n (%) yes | 43 (37.4) | 74 (50.0) | 117 (44.5) |
Chi-square/t tests indicate significant differences by diabetes type: * P < 0.05; ** P < 0.01
BG blood glucose, SD standard deviation, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus
aIncludes only those who reported missed work time in the past week (n = 71)
A majority of working respondents (70.7%) also reported that they experienced any kind of work productivity issues due to this last episode of PPH. More specifically, 9.5% reported missing work meetings or appointments, 11.0% reported canceling and rescheduling a work meeting or appointment, 27.8% indicated that they made more mistakes at work, 43.7% needed to take a break at work, 54.4% found it difficult to focus, and 44.5% indicated being less productive at work due to PPH.
There were some significant differences in work productivity issues due to PPH by diabetes type and by country. Respondents with T2DM were significantly more likely to report having any work productivity issues due to PPH (77.0%) compared to those with T1DM (62.9%, P < 0.05). Additionally, respondents with T2DM were significantly more likely to report that they found it difficult to focus due to PPH (62.8%) compared to those with T1DM (43.5%, P < 0.01). Respondents with T2DM were also significantly more likely to indicate that they were less productive at work due to PPH (50.0%) compared to those with T1DM (37.4%, P < 0.05). Respondents in Germany were significantly more likely to report any work productivity issues due to PPH (83.5%) compared to those in the USA (64.5%) and UK (61.6%, P < 0.01). Compared to respondents in the USA and UK, respondents in Germany were also significantly more likely to report that they made more mistakes at work (Germany, 37.1%; USA, 24.7%; UK, 19.2%, P < 0.05) and found it difficult to focus (Germany, 64.9%; USA, 46.2%; UK, 50.7%, P < 0.05) due to PPH.
The impact of PPH on missed work time and reduced work functioning/productivity is shown in Table 3.
PPH and BG Measurement
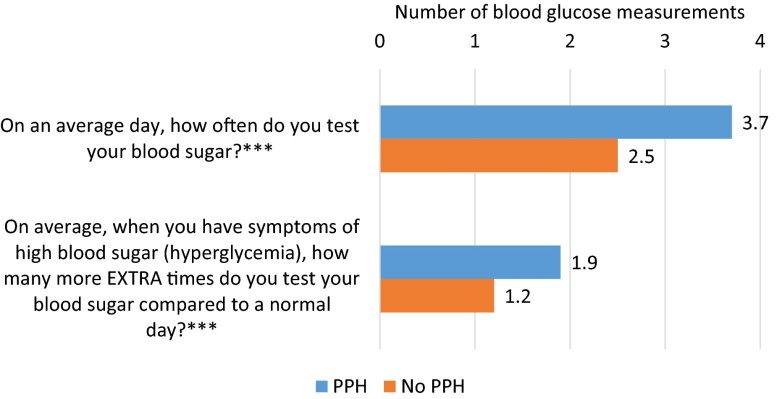
Respondents reported measuring BG 3.2 times on an average day and an average of 1.6 extra times compared to a normal day on days they experience symptoms of hyperglycemia. There were significant differences in frequency of BG measurement by whether or not respondent experienced PPH in the past week (Fig. 1). Respondents who experienced PPH in the past week reported measuring their BG a significantly greater number of times on an average day compared to respondents who did not experience PPH in the past week (3.7 vs. 2.5, P < 0.001). This is a difference of 1.2 tests per day, which translates into approximately 438 additional BG tests per person per year associated with PPH. Further, respondents who experienced PPH in the past week also reported that they measure their BG a significantly greater number of extra times when they experience symptoms of hyperglycemia compared to those who did not experience PPH in previous week (1.9 vs. 1.2, P < 0.001). This suggests that those who experienced PPH measure their BG more frequently than those without PPH in the previous week both in general, on an average day, and when they experience symptoms of hyperglycemia. Results were similar by diabetes type and by country.
Fig. 1.
Blood glucose measurement by experienced PPH in past week (n = 906). Note: t tests indicate significant differences by experience of PPH in past week (***P < 0.001). PPH post-prandial hyperglycemia
PPH and Healthcare Resource Utilization
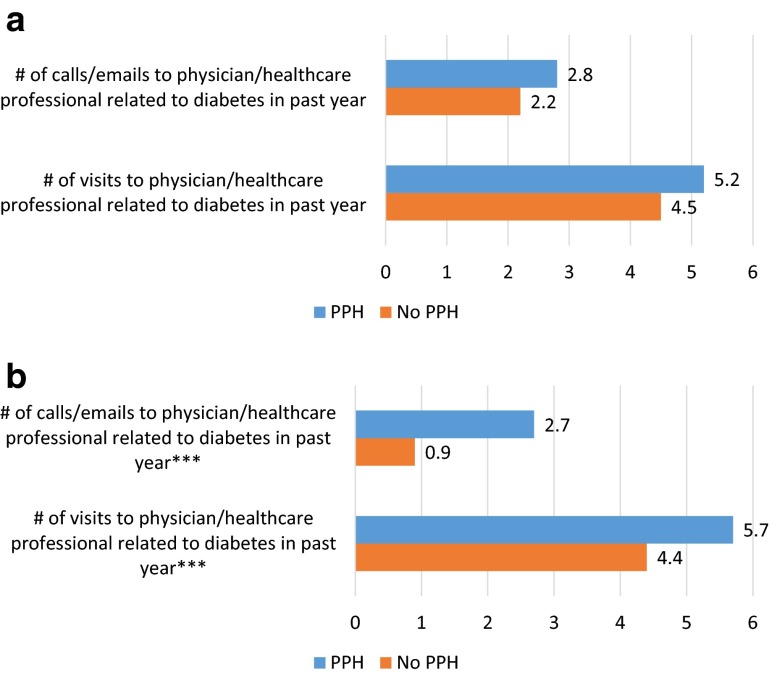
Respondents who experienced PPH were significantly more likely to use healthcare resources than those without PPH. Specifically, those with PPH in the past week visited a physician or other healthcare professional 5.5 times for diabetes in the past year, compared to 4.4 times (P < 0.001) among those who did not experience PPH. Additionally, respondents who experienced PPH in the past week called or emailed a physician or other healthcare professional for diabetes a significantly greater number of times in the past year than those without PPH (2.7 vs. 1.4, P < 0.001). The impact of PPH on healthcare resource utilization by diabetes type is presented in Fig. 2.
Fig. 2.
Healthcare contact related to diabetes by experienced PPH in the past week among people with a type 1 diabetes (n = 356) and b type 2 diabetes (n = 550). Note: Chi-square tests indicate significant differences by experience of PPH (***P < 0.001). PPH post-prandial hyperglycemia
As shown in Table 4, respondents who experienced PPH in the past week were also significantly more likely to report being diagnosed with one or more medical complications related to diabetes compared to those who did not experience PPH (71.7% vs. 54.5%, P < 0.001). The top three diabetes complications, all of which were significantly greater for those with PPH than those without, were high blood pressure (41.0% vs. 29.0%, P < 0.001), eye problems (33.3% vs. 21.2%, P < 0.001), and nerve damage/neuropathy (27.6% vs. 20.6%, P < 0.05).
Table 4.
Diagnosed medical complications related to diabetes by experienced post-prandial hyperglycemia (PPH) in past week and diabetes type
| T1DM (n = 356) | T2DM (n = 550) | Total (n = 906) | |||||
|---|---|---|---|---|---|---|---|
| PPH (n = 219) | No PPH (n = 137) | PPH (n = 342) | No PPH (n = 208) | PPH (n = 561) | No PPH (n = 345) | ||
| Diagnosed with one or more medical complications related to diabetes | n (%) yes | 131 (59.8) | 62 (45.3)** | 271 (79.2) | 126 (60.6)*** | 402 (71.7) | 188 (54.5)*** |
| High blood pressure | n (%) yes | 60 (27.4) | 30 (21.9) | 170 (49.7) | 70 (33.7)*** | 230 (41.0) | 100 (29.0)*** |
| Eye problems | n (%) yes | 84 (38.4) | 27 (19.7)*** | 103 (30.1) | 46 (22.1)* | 187 (33.3) | 73 (21.2)*** |
| Nerve damage (neuropathy) | n (%) yes | 44 (20.1) | 21 (15.3) | 111 (32.5) | 50 (24.0)* | 155 (27.6) | 71 (20.6)* |
Chi-square tests indicate significant differences by experience of PPH in past week: * P < 0.05; ** P < 0.01; *** P < 0.001
PPH post-prandial hyperglycemia, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus
There were some differences in results by diabetes type. The relationship between PPH and contact with healthcare professionals was significant among respondents with T2DM, but not among those with T1DM. The associations between experience of PPH and reported nerve damage/neuropathy and between experience of PPH and reported high blood pressure were significant among respondents with T2DM, but not among respondents with T1DM.
Results also differed somewhat by country. The association between PPH and visits to doctors/healthcare professionals for diabetes in the past year was not significant among German respondents. Additionally, PPH and diagnosis of one or more medical complications due to diabetes were not significantly related among respondents in the USA. The link between PPH and eye problems was not significant among respondents in the UK. PPH and nerve damage/neuropathy were only significantly associated for respondents in the USA. Further, high blood pressure was not significantly different between those with and without PPH among respondents in the UK.
The Economic Costs of PPH
Costs of PPH included missed work time, extra use of BG monitoring strips, and healthcare resource utilization.
Average annual costs per person for missed work time were estimated among employed respondents using reported hourly income, missed work time in the past week, and average work weeks per year in each country. Costs were 394.78 € in Germany (given 8.48 € per week; 40 work weeks), £396.83 in the UK (given £8.62 per week; 46 work weeks), and $606.30 USD in the USA (given $13.05 USD per week; 47 work weeks). For comparison purposes, the equivalent costs for all countries in USD were $520.80 in Germany, $609.50 in the UK, and $606.30 in the USA. Estimated average cost of missed work in the past week did not differ significantly by diabetes type or country.
Annual costs of additional BG test strips associated with PPH were estimated to be 51.10 € in Germany (given 0.20 € per BG test strip [25] and an average of 0.7 additional BG tests per day), £113.88 GBP in the UK (given £0.24 GBP per BG test strip [27] and 1.3 additional BG tests per day), and $500.78 USD in the USA (given $0.98 USD per BG test strip [26] and 1.4 additional BG tests per day). For comparison purposes, the equivalent costs for all countries in USD were $67.37 in Germany, $175.03 in the UK, and $500.78 in the USA.
Annual costs of additional physician office visits related to PPH were £210.00 GBP in the UK (2.0 additional visits; £105 GBP per visit [28]; equivalent cost in USD was $322.77) and $132.30 in the USA (0.9 additional visits; $147 per visit [19]). As the association between PPH and physician office visits for diabetes care was not significant among German respondents, office visit costs due to PPH were not estimated for Germany.
Altogether, estimated costs associated with PPH may be substantial. Average annual costs of missed work time, additional BG test strips, and physician office visits associated with PPH in USD were $1107.30 in the UK and $1239.38 in the USA per employed person. In Germany, the average annual cost of missed work time and additional BG test strips associated with PPH was $588.17 USD per employed person.
Cost estimates by diabetes type and country are shown in Table 5.
Table 5.
Estimated annual costs associated with PPH by country and diabetes type
| Germany | UK | USA | ||
|---|---|---|---|---|
| Missed work time | ||||
| Estimated annual cost per employed person of missed work due to out-of-range PPGa | T1DM | $672.00 | $763.14 | $354.38 |
| T2DM | $565.20 | $730.48 | $474.23 | |
| BG measurement | ||||
| Estimated annual cost of additional BG measurement associated with PPH per personb | T1DM | $101.79 | $254.00 | $683.60 |
| T2DM | $38.28 | $111.08 | $382.06 | |
| Physician/HCP office visits related to diabetes | ||||
| Estimated annual cost of additional physician/HCP office visits associated with PPH per personb,c | T1DM | – | $188.82 | $151.41 |
| T2DM | – | $451.88 | $126.42 | |
| Total estimated costs | T1DM | $773.79 | $1205.96 | $1189.39 |
| T2DM | $603.48 | $1293.44 | $982.71 | |
All costs reported in USD, using exchange rates from July 22, 2013
BG blood glucose, HCP healthcare professional, PPG post-prandial blood glucose, PPH post-prandial hyperglycemia, T1DM type 1 diabetes mellitus, T2DM type 2 diabetes mellitus
aEstimated using average number of work weeks in each country (Germany = 40, UK = 46, USA = 47)
bEstimates calculated using average local tariffs in each country, as reported in “Methods” section
cCosts not estimated for Germany due to non-significant association with PPH
Discussion
Consistent with prior research [2], this study has shown that PPH is a frequent occurrence among people with T1DM and T2DM. While there is much discussion in the literature about the economic costs of diabetes in general, there is little understanding of specific costs associated with PPH among people with diabetes. The results from this study suggest that there may be substantial economic costs associated with PPH as a result of a broad range of impacts including missed work time, decreased worker productivity, increased BG measurement costs, and more frequent office visits to healthcare professionals and reported medical complications related to diabetes. In addition to these short-term impacts and economic costs, there are additional costs that are more difficult to quantify and assign a specific dollar value that should also be included in considering the economic burden of PPH. These include increased worker presenteeism, which is known to lead to decreased work productivity [22, 30], costs of additional calls and emails to healthcare professionals, which although not usually billable, do increase healthcare resource utilization, and costs due to increased rates of medical complications. The top three complications reported more frequently by those experiencing PPH were high blood pressure, eye problems, and nerve damage/neuropathy.
In total, the estimated costs associated with PPH in this study may affect multiple groups as they are borne by patients, payers, the healthcare system, and employers. For example, increased costs of BG monitoring strips may be borne by either the patient as an out-of-pocket expense or by the payer, while increased physician or healthcare professional office visits are a cost to the healthcare system and payers. Thus, reducing costs associated with PPH would benefit society as a whole.
Results were generally similar for respondents with T1DM and T2DM, with some exceptions. Although the association between PPH and healthcare contact and between PPH and some medical complications did not reach statistical significance among those with T1DM, this may be due to the smaller sample size, or it may be that people with T1DM are more experienced in managing PPH due to their longer duration of diabetes and disease management, on average, compared to those with T2DM. Additionally, people with T2DM were significantly more likely than those with T1DM to report some work productivity issues due to PPH. It is possible that the severity of hyperglycemia might explain such differences, and this could be explored in future research. Some country differences were also evident, though explanations of such differences are beyond the scope of this study.
The study has several important limitations, which should be recognized. First, as with all surveys based on PROs, recall bias may have impacted results. The survey relied on patients to indicate their experiences with PPH and other outcomes, so it is possible that respondent recollections were inaccurate. Nevertheless, the survey used a recall period of 1 week for experience of PPH, which focus groups used to develop the survey indicated was appropriate. Selection bias may also have affected results. As with all studies relying on data from Internet surveys, respondents were drawn from panels of people who were required to be literate and have access to computers and the Internet. In the three countries studied, however, rates of literacy and internet use are both high. For instance, in the UK, the literacy rate is 99% [31], and approximately 83% of households had access to the internet in 2013 [32]. Additionally, given the cross-sectional, observational nature of the study, the results must be interpreted with caution. Causation may not be assumed in the associations found in the analyses. For instance, the association between PPH and healthcare contact among respondents does not necessarily mean that PPH causes more frequent doctor visits and calls/emails. There could be other unobserved factors associated with PPH that increase healthcare contact or additional BG measurement. Moreover, physician confirmation of respondent diabetes diagnosis and other clinical values was not possible due to the panel format of the internet survey. Thus, it is possible that some survey participants reported a diagnosis of diabetes when they had no such diagnosis in reality or reported their diabetes type inaccurately. It is unlikely, however, that such respondents were great enough in number to have an impact on the overall results. Potential respondents were not informed prior to the screener that only people with diabetes would be eligible to complete the survey. Clinical values, including BG measurements, were also not obtained. Such values would capture a more accurate measure of PPH, including the level of hyperglycemia, and may explain some differences in results by diabetes type. Last, further research is needed to explore other potential costs related to PPH and to estimate costs that are more difficult to measure. Future research could also explore how cross-country differences in factors such as diabetes care and treatment may affect the economic burden of PPH.
Conclusions
This is the first study to address an economic burden associated specifically with PPH among people with diabetes. The findings show that there are substantial costs associated with PPH, which should be included when calculating the cost of diabetes. These costs are the result of lost work productivity, increased diabetes management, and healthcare resource utilization. Reducing the incidence of PPH among people with diabetes would benefit not only patients but also payers, the healthcare system, and employers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was sponsored by Novo Nordisk. The article processing charges for this publication were funded by Novo Nordisk. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Meryl Brod and Kathryn M. Pfeiffer are paid consultants to the pharmaceutical industry, including Novo Nordisk. Annie Nikolajsen is an employee of Novo Nordisk A/S. James Weatherall is an employee of Novo Nordisk, Inc.
Compliance with Ethics Guidelines
Prior to commencement, the study received ethics approval from Copernicus Group Institutional Review Board (# TBG1-11-116). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all participants for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. Guideline for management of postmeal glucose in diabetes [Internet]. 2011 [cited Mar 27, 2014]. Available from: http://www.idf.org/2011-guideline-management-postmeal-glucose-diabetes.
- 3.Nathan DM, DCCT/EDIC Research Group The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. doi: 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriello A. The glucose triad and its role in comprehensive glycaemic control: current status, future management. Int J Clin Pract. 2010;64(12):1705–1711. doi: 10.1111/j.1742-1241.2010.02517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peter R, Rees A. Postprandial glycaemia and cardiovascular risk. Br J Diab Vasc Dis. 2008;8(1):8–14. doi: 10.1177/14746514080080010401. [DOI] [Google Scholar]
- 7.Lawton J, Rankin D, Cooke D, Elliott J, Amiel S, Heller S, et al. Patients’ experiences of adjusting insulin doses when implementing flexible intensive insulin therapy: a longitudinal, qualitative investigation. Diabetes Res Clin Pract. 2012;98(2):236–242. doi: 10.1016/j.diabres.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Aryangat AV, Gerich JE. Type 2 diabetes: postprandial hyperglycemia and increased cardiovascular risk. Vasc Health Risk Manag. 2010;6:145–155. doi: 10.2147/vhrm.s8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalot F, Pagliarino A, Valle M, Di Martino L, Bonomo K, Massucco P, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34(10):2237–2243. doi: 10.2337/dc10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91(3):813–819. doi: 10.1210/jc.2005-1005. [DOI] [PubMed] [Google Scholar]
- 11.Ceriello A. Cardiovascular effects of acute hyperglycaemia: pathophysiological underpinnings. Diab Vasc Dis Res. 2008;5(4):260–268. doi: 10.3132/dvdr.2008.038. [DOI] [PubMed] [Google Scholar]
- 12.Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: should we care? Diabetes Care. 2011;34(Suppl 2):S120–S127. doi: 10.2337/dc11-s206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzo MR, Marfella R, Barbieri M, Boccardi V, Vestini F, Lettieri B, et al. Relationships between daily acute glucose fluctuations and cognitive performance among aged type 2 diabetic patients. Diabetes Care. 2010;33(10):2169–2174. doi: 10.2337/dc10-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance, The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation. 2007;116(2):151–157. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 15.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283(19):2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 16.Lowe LP, Liu K, Greenland P, Metzger BE, Dyer AR, Stamler J. Diabetes, asymptomatic hyperglycemia, and 22-year mortality in black and white men. The Chicago Heart Association Detection Project in Industry Study. Diabetes Care. 1997;20(2):163–169. doi: 10.2337/diacare.20.2.163. [DOI] [PubMed] [Google Scholar]
- 17.Nakagami T, DECODA Study Group Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia. 2004;47(3):385–394. doi: 10.1007/s00125-004-1334-6. [DOI] [PubMed] [Google Scholar]
- 18.Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL. Postchallenge hyperglycemia and mortality in a national sample of US adults. Diabetes Care. 2001;24(8):1397–1402. doi: 10.2337/diacare.24.8.1397. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36(4):1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration (FDA). Guidance for industry, patient-reported outcome measures: use in medical product development to support labeling claims; 2009 [cited Dec 14, 2015]. Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. [DOI] [PMC free article] [PubMed]
- 21.Charmaz K. Premises, principles, and practices in qualitative research: revisiting the foundations. Qual Health Res. 2004;14(7):976–993. doi: 10.1177/1049732304266795. [DOI] [PubMed] [Google Scholar]
- 22.Endicott J, Nee J. Endicott Work Productivity Scale (EWPS): a new measure to assess treatment effects. Psychopharmacol Bull. 1997;33(1):13–16. [PubMed] [Google Scholar]
- 23.Organisation for Economic Co-operation and Development (OECD). Hours worked (indicator). [Internet]. 2014 [cited Dec 2, 2014]. Available from: http://www.oecd-ilibrary.org/employment/hours-worked/indicator/english_47be1c78-en.
- 24.US Bureau of Labor Statistics. Labor Force Statistics from the Current Population Survey (CPS), 2013; US [Internet]. 2013 [cited Dec 2, 2014]. Available from: http://www.bls.gov/cps/aa2013/cpsaat19.htm.
- 25.Lauer-Taxe. January 1, 2016. Lauer Fischer [cited Jan 12, 2016]. Available from: http://www2.lauer-fischer.de/produkte/lauer-taxe/lauer-taxe/.
- 26.Yeaw J, Lee WC, Aagren M, Christensen T. Cost of self-monitoring of blood glucose in the United States among patients on an insulin regimen for diabetes. J Manag Care Pharm. 2012;18(1):21–32. doi: 10.18553/jmcp.2012.18.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haymarket Medical Media. Monthly Index of Medical Specialties (MIMS). [Internet]. February 2015 [cited Jan 8, 2016]. Available from: http://www.mims.co.uk/.
- 28.UK Department of Health [Internet]. NHS reference costs 2013–2014. London, England: Department of Health; 2014 [cited Jan 7, 2016]. Available from: https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014.
- 29.Bohrnstedt GW, Knoke D. Statistics for social data analysis. 3. Itasca, Illinois: FE Peacock Publishers; 1994. [Google Scholar]
- 30.Koopman C, Pelletier KR, Murray JF, Sharda CE, Berger ML, Turpin RS, et al. Stanford presenteeism scale: health status and employee productivity. J Occup Environ Med. 2002;44(1):14–20. doi: 10.1097/00043764-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 31.US Central Intelligence Agency [Internet]. The world factbook: United Kingdom. Washington, DC: CIA; 2014 [cited Jul 17, 2014]. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/uk.html.
- 32.UK Office for National Statistics. Statistical bulletin: internet access—households and individuals, 08 August 2013. U.K.; 2013 [cited Jul 17, 2014]. Available from: http://www.ons.gov.uk/ons/rel/rdit2/internet-access-households-and-individuals/2013/stb-ia-2013.html#tab-Computer-and-Internet-use.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.