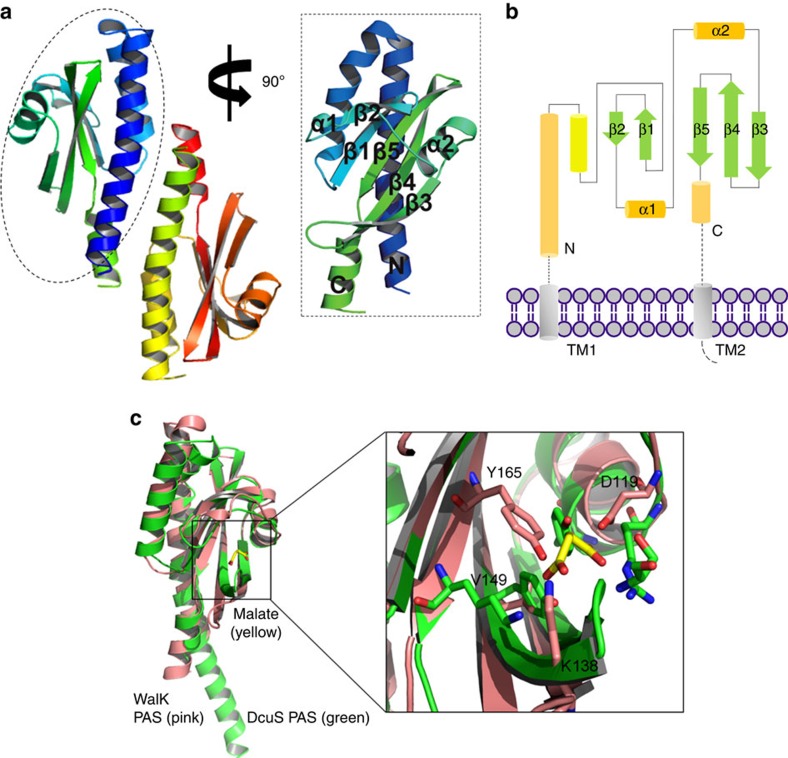
Figure 1. Structural characterization of erWalK reveals a canonical PAS domain.
(a) The asymmetric unit of the crystal of erWalK contains an antiparallel homodimer. (b) Topology diagram of the structure of erWalK reveals a canonical PAS domain, as the β-strands are arranged in the order of 2-1-5-4-3. (c) Tertiary structure overlay of erWalK with a well-studied PAS domain protein DcuS PAS (PDB code: 3BY8) reveals four possible signal-transduction residues in erWalK. The structures of erWalK and DucS PAS are coloured pink and green, respectively; the ligand of DcuS PAS D-malate is coloured yellow. The potential signal-transduction residues of erWalK are indicated.