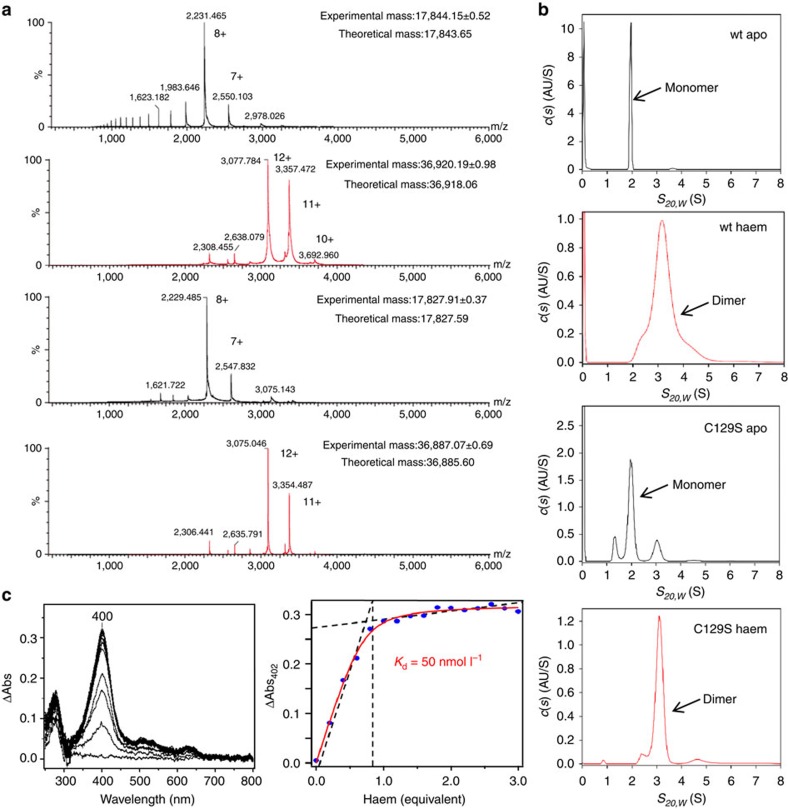
Figure 2. PGRCM1 is dimerized by binding with haem.
(a) Mass spectrometric analyses of the wild-type (wt) PGRMC1 or the C129S mutant in the presence or absence of haem under non-denaturing condition. Both proteins had identical lengths (a.a.44–195). Data of experimental mass show mean±s.d. (b) SV-AUC analyses of the wt-PGRMC1 and the C129S mutant (a.a.44–195) in the presence or absence of haem. SV-AUC experiments were performed with 1.5 mg ml−1 of PGRMC1 proteins. The major peak with sedimentation coefficient S20,w of 1.9∼2.0 S (monomer) or 3.1 S (dimer) was detected. (c) Difference absorption spectra of PGRMC1 (a.a.44–195) titrated with haem (left panel). The titration curve of haem to PGRMC1 (right panel). The absorbance difference at 400 nm is plotted against the haem concentration.