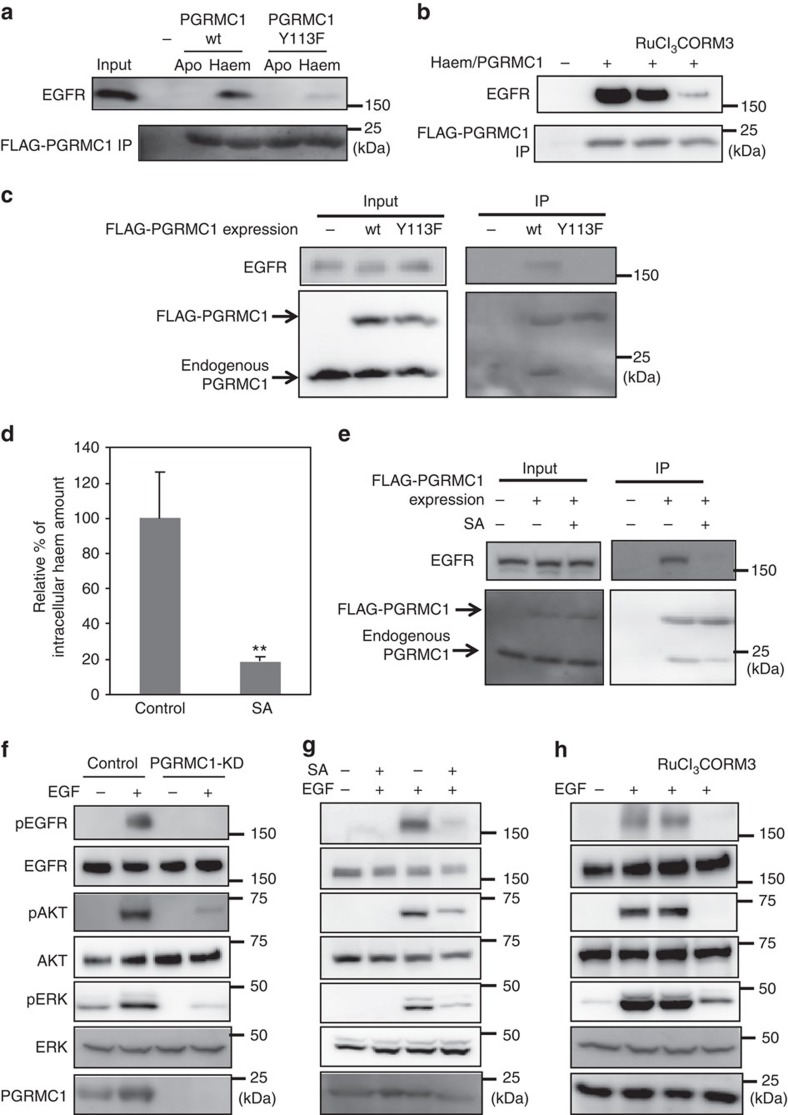
Figure 4. Haem-dependent dimerization of PGRMC1 is necessary for tumour proliferation mediated by EGFR signalling.
(a) FLAG-PGRMC1 wild-type (wt) and Y113F mutant proteins (a.a.44–195), in either apo- or haem-bound form, were incubated with purified EGFR and co-immunoprecipitated with anti-FLAG antibody-conjugated beads. Input and bound proteins were detected by Western blotting. (b) In vitro binding assay was performed as in (a) using haem-bound FLAG-PGRMC1 wt (a.a.44–195) and purified EGFR with or without treatment of RuCl3 and CORM3. (c) FLAG-PGRMC1 wt or Y113F (full length) was over-expressed in HCT116 cells and immunoprecipitated with anti-FLAG antibody-conjugated beads. Co-immunoprecipitated proteins (FLAG-PGRMC1, endogenous PGRMC1 and EGFR) were detected with Western blotting by using anti-PGRMC1 or anti-EGFR antibody. (d) HCT116 cells were treated with or without 250 μmol l−1 of succinylacetone (SA) for 48 h. The intracellular haem was extracted and quantified by reverse-phase HPLC. The data represent mean±s.d. of four separate experiments. **P<0.01 using unpaired Student's t-test. (e) Co-immunoprecipitation assay was performed as in (c) with or without SA treatment in HCT116 cells. (f) HCT116 cells expressing control shRNA or those knocking down PGRMC1 (PGRMC1-KD) were treated with EGF or left untreated, and components of the EGFR signaling pathway were detected by Western blotting. (g,h) HCT116 cells were treated with or without EGF, SA, RuCl3 and CORM3 as indicated, and components of the EGFR signaling pathway were detected by Western blotting.