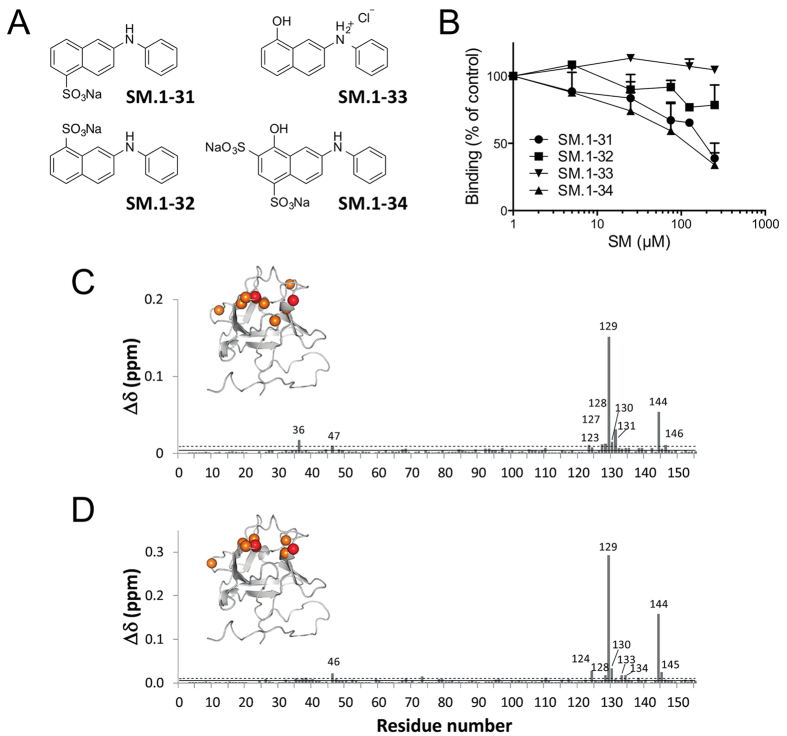
Figure 6.
(A) Structure of the 4 phenylamino-substituted naphthalenes synthesized and analyzed in the present study. (B) Antiproliferative activity of the four anilino-naphthalenes was analyzed as in Fig. 4A. Data are the percentage of control proliferation (in absence of molecules). (C) Graphical representation of the combined 1HN and 15N FGF2 chemical shift perturbation determined for the various residues, according to36
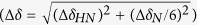 , following the addition of phenylamino-substituted naphthalenes. C) Chemical shift perturbation (CSP) induced by SM.1–34 addition in SM:FGF2 1:1 stoichiometric ratio D) CSP induced by SM.1–31 addition in SM:FGF2 1:3 stoichiometric ratio. The continuous and dashed lines represent the average and the average plus a standard deviation (SD) values, respectively. Residues affected by CSP > (<CSP>) + 1SD are mapped on the FGF2 structures as orange spheres. Residues Arg129 and Lys144, showing the highest CSP, are shown as red spheres.
, following the addition of phenylamino-substituted naphthalenes. C) Chemical shift perturbation (CSP) induced by SM.1–34 addition in SM:FGF2 1:1 stoichiometric ratio D) CSP induced by SM.1–31 addition in SM:FGF2 1:3 stoichiometric ratio. The continuous and dashed lines represent the average and the average plus a standard deviation (SD) values, respectively. Residues affected by CSP > (<CSP>) + 1SD are mapped on the FGF2 structures as orange spheres. Residues Arg129 and Lys144, showing the highest CSP, are shown as red spheres.