Abstract
Background
Streptococcus pneumoniae is responsible for approximately 1.6 million yearly deaths worldwide. An up-to-date evidence base on the effects of pneumococcal conjugate vaccines (PCVs) on infectious diseases and mortality in any population or setting regardless of age or health status is currently lacking.
Methods
We systematically searched MEDLINE and EMBASE for pertinent randomized controlled trials (RCTs). Two reviewers independently screened 9498 titles/abstracts and 430 full-text papers for eligible trials. The outcomes of our meta-analysis were pooled using relative risks (RRs) with a random effects model or Peto’s odds ratios (ORs) if event rates were <1%.
Results
21 RCTs comprising 361 612 individuals were included. PCVs reduced the risk for invasive pneumococcal disease (odds ratio [OR]: 0.43, 95% confidence interval [CI]: [0.36; 0.51]), all-cause acute otitis media (AOM) (RR: 0.93, 95% CI: [0.86; 1.00]), pneumococcal AOM (RR: 0.57, 95% CI: [0.39; 0.83]), all-cause pneumonia (RR: 0.93, 95% CI: [0.89; 0.97]), and pneumococcal pneumonia (RR: 0.78, 95% CI: [0.62; 0.97]). We found no significant effect of PCVs on all-cause mortality (RR: 0.95, 95% CI: [0.88; 1.03]) or recurrent AOM (RR: 0.87, 95% CI: [0.72; 1.05]).
Conclusion
PCVs are associated with large risk reductions for pneumococcal infectious diseases, smaller risk reductions for infectious diseases from any cause, and no significant effect on all-cause mortality.
Streptococcus pneumoniae (Pneumococcus) is globally recognized as one of the most important pathogens causing considerable morbidity and mortality (1). The World Health Organization (WHO) estimates that Pneumococcus is related to 1.6 million yearly deaths worldwide, most of them occurring in low-income countries (1, 2). Children under 2 years of age, the elderly, and people with immunodeficiency are at highest risk (1– 3).
There are at least 90 different pneumococcal serotypes but most diseases are caused by fewer than 30 (4). The first pneumococcal vaccines contained capsular polysaccharides from the most common infective serotypes of the bacterium (pneumococcal polysaccharide vaccines, PPVs). PPVs fail to induce an adequate immune response in infants. As a consequence, pneumococcal conjugate vaccines (PCVs) were developed by linking pneumococcal capsular material to carrier proteins (5). The first PCV contained 7 serotypes (PCV7) that were responsible for 65–80% of severe pneumococcal diseases in industrialized countries (4).
Previous systematic reviews (6– 13) evaluated the evidence base for PCVs in the prevention of infectious diseases but only few performed meta-analyses (6, 7). A recent systematic review (10) focused exclusively on the prevention of otitis media as an outcome in under 12-year-olds. PCVs have already been extensively studied (14– 17) and they were declared safe by the WHO (18). However, an up-to-date summary and meta-analysis of the effectiveness of PCVs on a broad range of patient-centered outcomes in a variety of populations is currently lacking.
We conducted a comprehensive systematic review and meta-analysis to quantify the effects of PCVs in any population independent of age or health status compared with placebo, no vaccination, or other non-pneumococcal conjugate vaccines on all-cause mortality and all-cause, pneumococcal, and vaccine-specific infectious diseases.
Methods
A protocol was registered with the Centre for Reviews and Dissemination (CRD42014009005) (19).
Eligibility criteria and outcomes
We considered randomized controlled trials (RCTs) which compared PCVs given alone or in combination with other vaccines versus placebo, no intervention or other, non-pneumococcal conjugate, vaccines in any population irrespective of age or health status. At least one primary outcome (all-cause mortality, pneumonia, acute otitis media [AOM], invasive pneumococcal disease [IPD]) or secondary outcome (same as primary outcomes but caused by vaccine-specific serotypes) had to be reported.
Search methods
We systematically searched Medline and EMBASE from their inception (MEDLINE, 1946; EMBASE, 1947) to October 2014 for eligible RCTs regardless of language and publication status. The search strategy (see eSupplement 1) was developed in collaboration with an experienced research librarian.
In addition, we handsearched reference lists of eligible articles and systematic reviews on the topic, contacted experts in the field, and searched the WHO trial registry platform (http://apps.who.int/trialsearch) for ongoing or unpublished eligible studies.
Study selection and data extraction
Working in pairs, investigators (HE, VG, DV, VK, AZ) independently screened titles and abstracts of retrieved references to identify potentially eligible publications. If one of the investigators judged a publication as potentially eligible, full text articles were obtained and assessed independently by two investigators (HE, VG).
We extracted outcome data based on clinical diagnosis; we preferred per-patient-data based on the intention-to-treat principle. Studies which exclusively reported AOM as number of episodes were excluded. In IPD, we made the assumption that the number of episodes equals the number of patients. One investigator (HE) extracted data using a pilot-tested data abstraction form and assessed risk of bias (20). This was checked by another investigator (VG). Any disagreements were resolved by consensus or third party arbitration (MB).
Data synthesis and analysis
We calculated relative risks (RRs) with corresponding 95% confidence intervals (CIs) using a random effects model to take into account clinical heterogeneity (21). For outcomes with event rates below 1%, we calculated Peto’s odds ratios (ORs) which are more appropriate in such cases (20). The relative effect estimates can be applied to specific baseline risks of different settings to calculate corresponding absolute effects. We present a worked example in the discussion using numbers needed to treat (20).
Our research question was relatively broad to give readers a comprehensive overview of the topic. Therefore, we thoroughly assessed between-study heterogeneity by visual inspection of forest plots and the degree of inconsistency (I2) (20). In case of moderate or high inconsistency (I2≥ 30%), we conducted subgroup and sensitivity analyses to find explanations for the detected heterogeneity and present results accordingly (20).
We assessed publication bias using funnel plots for outcomes for which at least 10 studies were available (22). All statistical analyses were performed using Stata 13.1.
Results
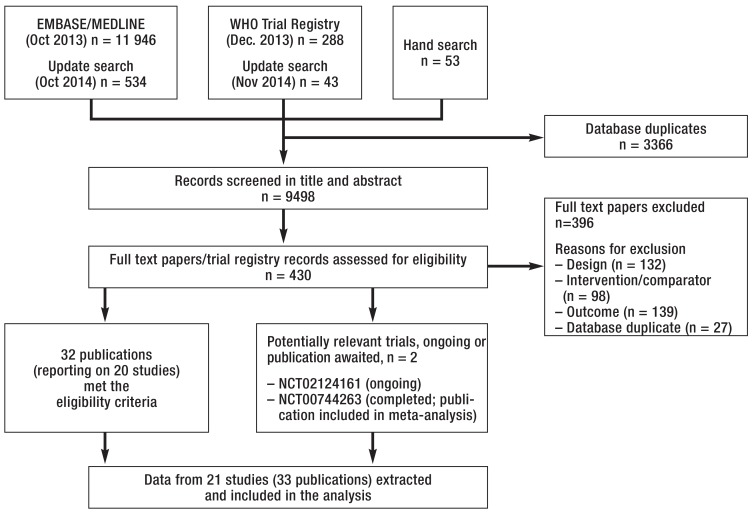
We screened 9498 titles and abstracts, assessed 430 full texts, and included 21 RCTs (Figure 1). A list of all included studies with corresponding references is provided in eSupplement 2.
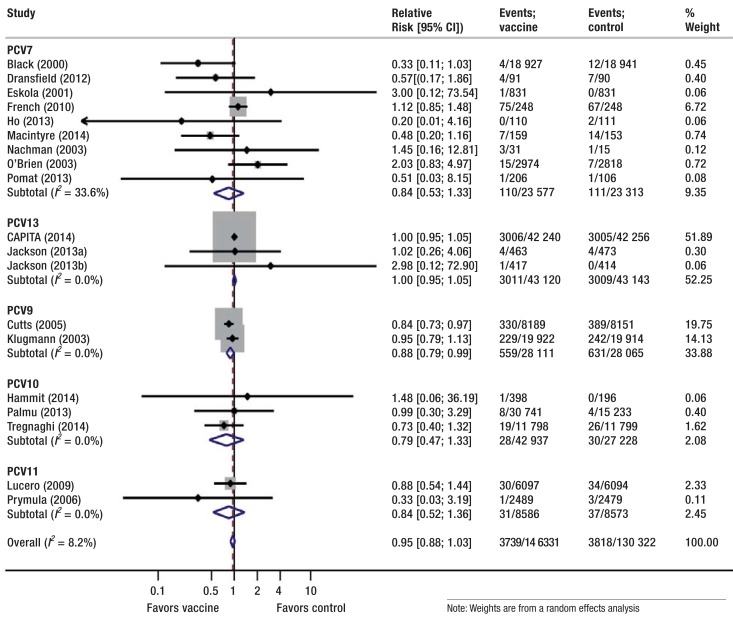
Figure 1.
Effect of pneumococcal conjugate vaccines versus control on all-cause mortality, stratified by vaccine type, Control comprises placebo, no intervention, 23-valent pneumococcal polysaccharide vaccine, and non-pneumococcal vaccines. PCV, pneumococcal conjugate vaccine; CI, confidence interval.
Full references for these studies can be found in eSupplement 2
General study characteristics
Patients were recruited between 1995 and 2008, the maximal follow-up period varied from 0.5 to 4 years (eTable 1). The 21 included RCTs were characterized as follows:
eTable. Study characteristics.
| Study | Country | Recruitment period | Populationhealth status | Maximal follow-up | Comparison | Dose schedule |
|---|---|---|---|---|---|---|
| Black (2000) |
USA | Oct 1995 – Aug 1998 | 0 to 2-year-olds Immunocompetent |
Until April 1999 | PCV7 vs meningococcus type C conjugate vaccine | At 2, 4, 6 and 12–15 months of age |
| CAPITA (2014) |
Netherlands | Sep 2008 – Jan 2010 | Over 60-year-olds Immunocompetent |
3.97 years | PCV13 vs placebo | 1 dose |
| Cutts (2005) |
Gambia | Aug 2000 – Feb 2003 | 0 to 2-year-olds Immunocompetent |
Until 2.5 years of age | PCV9 vs placebo | At 11, 17 and 24 weeks of age |
| Dransfield (2012) |
USA | Apr 2007 – May 2009 | Over 60-year-olds COPD, chronically ill Immune status unclear |
2 years | PCV7 vs PPV23 | 1 dose |
| Eskola (2001)*1 |
Finland | Dec 1995 – Apr 1997 | 0 to 2-year-olds Immune status unclear |
Until 2 years of age | PCV7 vs Hepatitis B vaccine | At 2, 4, 6, and 12 months of age |
| French (2010) | Malawi | Feb 2003 – May 2007 | 16 to 59-year-olds with previous IPD Immunodeficient (88.5% HIV positive) |
4.7 years | PCV7 vs placebo | 2 doses (4-week interval) |
| Gisselsson- Solen (2011) |
Sweden | Mar 2003 – Jun 2007 | 0 to 2-year-olds with previous AOM Immunocompetent |
Until 2 years of age | PCV7 vs no vaccine | 2+1 (if age ≥6 months); 3+1 (if age <6 months) doses |
| Hammit (2014) |
Kenya | Jan 2010 – Sep 2010 | 1 to 5-year-olds Immunocompetent |
0.6 years | PCV10 vs Hepatitis A | 2 doses + standard vaccine |
| Ho (2013) |
Brazil | Oct 2005 – May 2009 | 16 to 59-year-olds Immunodeficient (HIV positive) |
0.5 years | PCV7 +PPV23 vs Placebo + PPV23 | 1 dose |
| Jackson (2013a) |
USA | Not reported | Over 60-year-olds Immunocompetent |
1 year | PCV13 vs PPV23 | 1 dose |
| Jackson (2013b) |
USA | Not reported | Over 60-year-olds Immunocompetent |
1 year | PCV13 vs PPV23 | 1 dose |
| Klugman (2003) |
South Africa | Mar 1998 – Oct 2000 | 0 to 2-year-olds Immunocompetent (6.5% HIV positive) |
3.7 years | PCV9 vs placebo | 6, 10 and 14 weeks of age |
| Lucero (2009) |
Philippines | Jul 2000 – Dec 2003 | 0–2-year-olds Immunocompetent |
Until 2 years of age | PCV11 vs placebo | 6, 10 and 14 weeks of age |
| Macintyre (2014) |
Australia | May 2005 – Dec 2007 | Over 60-year-olds, hospitalized Immune status unclear |
1 years | PCV7 + PPV23 vs PPV23 | 1 dose |
| Nachman (2003) |
USA | Jan 1996 – Jan 1998 *2 | 0 to 2-year-olds Immunodeficient (HIV positive) |
Until 2 years of age | PCV7 vs placebo | 0, 8 , and 16 weeks after enrollment, and at 15 months of age |
| O’Brien (2003)*3 |
USA | Apr 1997 – Dec 1999 | Native American 0 to 2-year-olds Immune status unclear |
Until May 2000 | PCV7 vs Neisseria meningitidis group C protein conjugate vaccine | At 2, 4, 6 and 12–15 months of age |
| Palmu (2013) |
Finland | Feb 2009 – Oct 2010 | 0 to 2-year-olds Immune status unclear |
2.9 years | PCV10 vs Hepatitis A or B vaccine | 3+1 or 2+1 doses (if age <7 months), 2+1 doses (if age 7–11 months), 2 doses (if age 12–18 months) |
| Pomat (2013) |
Papua New Guinea | May 2005 – Sep 2007 | 0 to 2-year-olds Immunocompetent |
1.5 years | PCV7+PPV23 vs no PCV7+PPV23 |
At birth, 1 and 2 months of age (neonatal group) or at ages 1, 2 and 3 months (infant group); PPV23 at 9 months for all |
| Prymula (2006) | Czech Republic, Slovakia | Oct 2000 – Sep 2002 | 0 to 2-year-olds, no acute illness Immune status unclear |
Until 2.3 years of age | PCV11 vs Hepatitis A vaccine | At 3, 4, 5 and 12–15 months of age |
| Tregnaghi (2014) | Argentina, Colombia, Panama | Jun 2007 – Dec 2008 | 0 to 2-year-olds Immunocompetent |
2.75 years | PCV10 vs Hepatitis B vaccine with Hepatitis A vaccine as booster | At 2, 4, 6 and 15–18 months of age |
| Veenhoven (2003) |
Netherlands | Apr 1998 – Jan 2001 | 0 to 7 year-olds with ≥2 AOM episodes 1 year before study entry Immunocompetent |
2.6 years | PCV7 + PPV23 vs Hepatitis A or B vaccine |
1 dose (if age 25–84 months); 2 doses (if age 12–24 months); followed by PPV23-booster |
*1Originally 3 study groups: PCV7 with nontoxic diphtheria-toxin analogue CRM197 carrier vs PCV7 meningococcal outer membrane protein complex conjugate vaccine carrier vs Hepatitis B vaccine; only PCV7 CRM197 vs Hepatitis B were analyzed
*2Randomization period
*3Only the results from one of three age groups (primary efficacy group, age 6 weeks to 7 months) Full references for these studies can be found in eSupplement 2
12 studies in under 2-year-olds,
Two studies in 2- to 15-year-olds,
Two studies in 16- to 59-year-olds, and
5 studies in over 60-year-olds.
Participating children were immunocompetent (7 RCTs), from the general population with not further specified immune status (6 RCTs), or immunodeficient (1 RCT). The participating 16- to 59-year-olds were immunodeficient (2 RCTs), and the over 60-year-olds were immunocompetent or of unclear immune status (2 RCTs). PCV7 was investigated in 11 studies, PCV10 and PCV13 in 3 studies, PCV9 and PCV11 in 2 studies. The under 2-year-olds received 3 or more doses including a booster (10 RCTs). One or 2 doses were given in 8 studies including the 7 studies in adults. Three studies used varying doses depending on age at baseline. In 4 studies, PPV23 was given as a booster to PCV7. Comparators were placebo or no vaccine (8 RCTs), hepatitis A or B vaccine (6 RCTs), a meningococcal vaccine (2 RCTs) and PPV23 (5 RCTs). Details on setting, eligibility criteria, co-administered vaccines, and outcome definitions in included studies are presented in eSupplement 3.
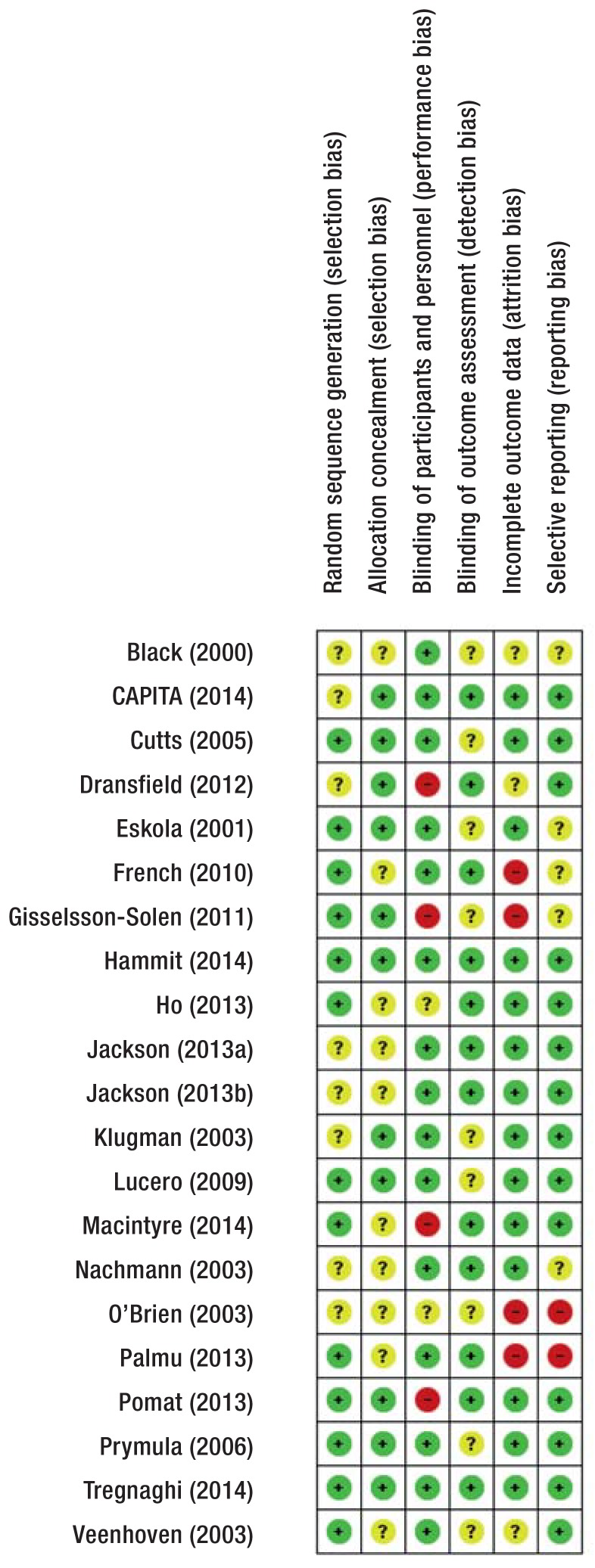
Risk of bias assessment
Random sequence generation was adequate in 13/21 studies (eFigure 2). Concealment of treatment allocation was adequate in 11/21 studies. Participants and personnel were blinded in 15/21 studies, the outcome assessors in 12/21. Incomplete outcome data was adequately addressed in 12/21 studies. We found evidence for selective outcome reporting in 2/21 studies. Three trials were stopped early (23– 25); Black et al. (23) was the only trial stopped early for benefit; it therefore carries a high risk for overestimation of effects (26).
eFigure 2.
Risk of bias assessment: review authors’ judgments for each included study
Green circle with plus sign: low risk of bias; yellow circle with question mark: unclear risk of bias; red circle with minus sign: high risk
We did not find evidence for publication bias with respect to all-cause mortality (eSupplement 4); for other outcomes assessment of funnel plots was limited by the small number of studies.
Outcomes
We did not find a statistically significant effect of PCVs on all-cause mortality (RR = 0.95, 95% CI [0.88; 1.03]; p = 0.2; I2= 8%; 19 studies pooled) (Figure 1).
When excluding trials of PPV23 administered in any form from sensitivity analysis (27– 31), results remained similar (RR = 0.95, 95% CI [0.87; 1.04]; I2= 18%). In under or over 60-year-olds (28– 33), the results were RR = 0.91 (95% CI [0.82; 1.00]) and RR = 1.0 (95% CI [0.95; 1.05]), respectively (p = 0.086 for interaction).
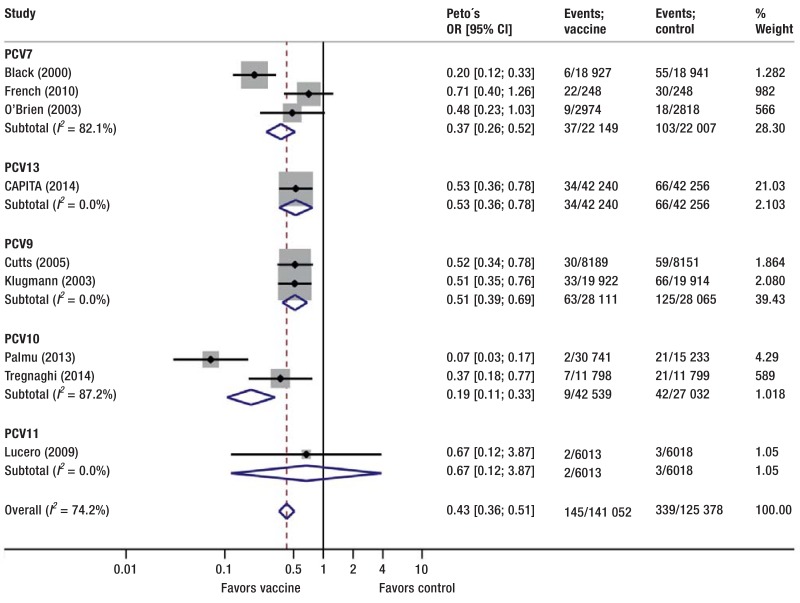
PCVs reduced the risk for IPD (OR = 0.43, 95% CI [0.36; 0.51]; p < 0.001; I2= 74%; 9 studies pooled) (Figure 2). The risk for vaccine-specific IPD was reduced too (OR = 0.27, 95% CI [0.22; 0.34]; p < 0.001; 10 studies pooled; I2= 58%) (eSupplement 5).
Figure 2.
Effect of pneumococcal conjugate vaccine versus control on invasive pneumococcal disease, stratified by vaccine type. Control comprises placebo, no intervention, 23-valent pneumococcal polysaccharide vaccine, and non-pneumococcal vaccines OR, odds ratio; CI, confidence interval
Full references for these studies can be found in eSupplement 2
In sensitivity analyses, we excluded one study not reporting first episodes but overall episodes (34), resulting in similar risk reductions but lower heterogeneity (IPD, OR = 0.46, 95% CI [0.38; 0.55]; I2= 51%). After additional exclusion of the study that stopped early for benefit and potentially overestimated effects (35), we no longer found evidence for heterogeneity (IPD, OR = 0.52, 95% CI [0.43; 0.64]; I2= 0%).
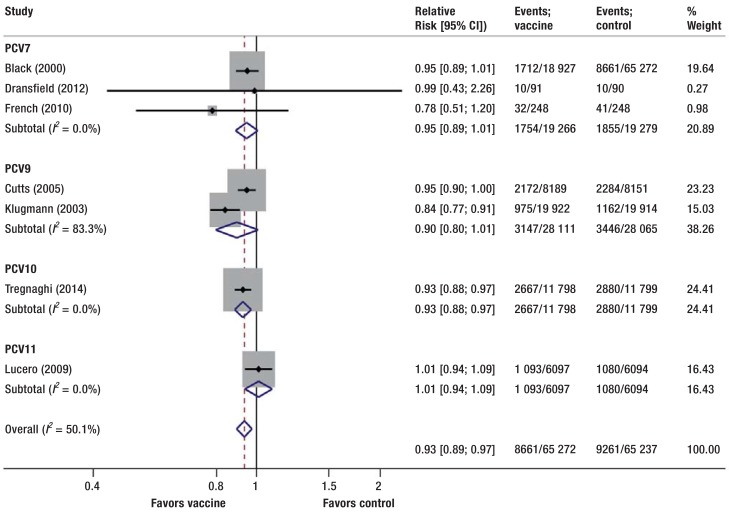
PCVs reduced the risk for all-cause pneumonia (RR = 0.93, 95% CI [0.88; 0.97]; p = 0.002; I2= 50%; 7 studies pooled) (Figure 3). One RCT reported pneumococcal and vaccine-specific pneumonia showing a reduced risk with PCVs (RR = 0.78, 95% CI [0.62; 0.97] and RR = 0.63, 95% CI [0.47; 0.85], respectively).
Figure 3.
Effect of pneumococcal conjugate vaccine versus control on pneumonia stratified by vaccine type. Control comprises placebo, no intervention, 23-valent pneumococcal polysaccharide vaccine, and non-pneumococcal vaccines
Full references for these studies can be found in eSupplement 2
In a sensitivity analysis, we excluded the RCT stopped early for benefit (35). This did not explain the detected heterogeneity (I2= 58%). Visual inspection of the forest plot suggested that the RCT by Klugmann et al. (36) contributed substantially to the heterogeneity. Its exclusion explained the detected heterogeneity, while the summary effect remained almost unchanged (RR = 0.95, 95% CI [0.92; 0.97]; I2= 0%).
PCVs reduced the risk for all-cause AOM (RR = 0.93, 95% CI [0.86; 1.00]; p = 0.038; I2= 28%; 3 studies pooled). Two studies reported pneumococcal AOM (RR = 0.57, 95% CI [0.39; 0.83], p = 0.003, I2= 11%). PCVs also significantly reduced the risk for vaccine-specific AOM (RR = 0.51, 95% CI [0.43; 0.60], p<0.001, I2= 0%, 5 studies pooled, eSupplement 5). All-cause recurrent AOM was reported by 2 studies (RR = 0.87, 95% CI [0.72; 1.05], p = 0.135; I2= 7%).
Discussion
This systematic review showed that PCVs reduced the risk for the following infectious diseases:
IPD
vaccine-specific IPD
all-cause AOM
pneumococcal and vaccine-specific AOM
pneumococcal and vaccine-specific pneumonia
all-cause pneumonia.
In general, there were large effects on vaccine-specific outcomes while effects on infectious diseases from any cause were rather modest. We found no significant effect of PCVs on all-cause mortality or recurrent AOM, although there was a trend towards a risk reduction for both outcomes. A post-hoc subgroup analysis suggested that the trend towards a reduction in mortality was limited to under 60-year-olds. More data is needed to clarify these trends.
Incidences of pneumococcal diseases vary widely due to differences in study settings and populations, e.g. for community acquired pneumonia in Europe between 1.6 and 11.6 per 1000 or IPD between 11 and 27 per 100 000 (18, 37). Putting our relative effect estimates in context, we estimated for example a number needed to treat (NNT) with PCV of 2857 to prevent one case of pneumonia (approximated yearly incidence of 0.5% in Germany [38,]), an NNT of 175 439 children to prevent one case of IPD (approximated incidence of IPD in children in Germany from 2010 of 0.01% [39, 40,]), or an NNT of 79 for AOM (approximated yearly incidence of 18% for children under 5 years of age [e1]).
Strengths and limitations
This systematic review and meta-analysis comprehensively looked at all PCVs in any population and setting, and aimed to identify all relevant RCTs. We decided on a broad scope to give readers an informative overview of the available evidence on various patient-relevant outcomes. The inherent clinical heterogeneity (different PCVs, populations, settings) was carefully considered in stratified analyses. We examined the statistical heterogeneity in all meta-analyses, and, if detected, empirically explored underlying reasons to explain that heterogeneity. In most of our meta-analyses, we found little or no heterogeneity across included RCTs despite our broad inclusion criteria. However, we detected substantial heterogeneity in meta-analyses for IPD, vaccine-specific IPD, and all-cause pneumonia. Exclusion of two studies, one for which we approximated the number of first episodes using the number of overall episodes (34) and one that stopped early for benefit (35) likely overestimating the effect size (26), explained all of the heterogeneity in meta-analyses for IPD and vaccine-specific IPD. In the meta-analysis for all-cause pneumonia, such a sensitivity analysis did not explain the detected heterogeneity. In a further exploratory analysis, we were able to identify the RCT by Klugmann et al. as the source of the heterogeneity but we could not determine any specific feature that made it different from the other RCTs. The effect estimates of these meta-analyses remain credible, because we identified the sources of heterogeneity and results hardly changed when excluding the RCT causing the heterogeneity in sensitivity analyses. Detailed trial characteristics in eTable 1 in conjunction with the methodological quality assessment and forest plots enable readers to see which type of PCV, population, and setting contributed to which evidence summary.
Overall, the risk of selection, performance, and detection bias were low or unclear in the included trials. There was some evidence for performance, attrition and reporting bias but results were robust in sensitivity analyses (data not shown). We did not find any evidence for publication bias for mortality; however, we cannot exclude publication bias because for most outcomes funnel plot analyses were not appropriate due to the small number of studies.
Herd effect and serotype replacement may have influenced our results. The herd effect decreases the risk of infection in both the vaccinated and the unvaccinated by reducing transmission and colonization in the nasopharynx of vaccine-specific Pneumococci (e2). This leads to smaller differences in effect estimates between intervention and control groups as the control group benefits from an indirect protective effect of the vaccination. In our review, however, we found large effects of PCVs on most vaccine-specific outcomes. After the suppression of vaccine-specific serotypes, the prevalence of non-vaccine-specific serotypes and non-pneumococcal pathogens can increase due to serotype replacement (e2). This can contribute to smaller effects on more generic outcomes such as all-cause pneumonia or all-cause AOM rather than on vaccine-specific outcomes. Indeed, our review found larger effects of PCVs on vaccine-specific AOM or vaccine-specific pneumonia than on all-cause AOM or all-cause pneumonia.
Comparison with other systematic reviews
There are several previous systematic reviews on PCVs (6, 7, 9, 13, e3, e4), of which only three conducted meta-analyses (6, 7, 13) and only two focused on RCTs (6, 7). These two compared PCV with placebo or another vaccine in under 2-year-olds. Their results were similar to ours (results only from the more recent one [7,]) for:
IPD (RR = 0.38, 95% CI [0.22; 0.67])
Vaccine-specific IPD (RR = 0.20, 95% CI [0.12; 0.34])
Pneumonia (RR = 0.94, 95% CI [0.9; 0.98]).
In addition, this recent meta-analysis (7) reported similar effect sizes for:
AOM reported as overall episodes (RR = 0.86, 95% CI [0.68; 1.00])
Vaccine-specific AOM (RR = 0.43, 95% CI [0.37; 0.5])
Pneumococcal AOM (RR = 0.63, 95% CI [0.51; 0.79])
Recurrent AOM (RR = 0.9, 95% CI [0.84; 0.96]).
Overall, these results are consistent with ours; small differences may be due to inconsistent definitions of AOM, differences in the count (overall episodes versus first episodes) and the number of RCTs included.
A meta-analysis of observational studies which compared the incidence of IPD cases related to vaccine serotypes before and after the introduction of a 2+1 PCV7 schedule (13) suggested PCV7 to be effective on IPD cases related to vaccine serotypes for under-2-year-olds (rate ratio = 0.1, 95% CI [0.04; 0.3]).
A more recent systematic review with a literature search from 2010 was published over several articles (11, 12, e5– e10) and addressed all-cause and pneumococcal pneumonia, vaccine-specific IPD, vaccine-specific carriage, immunogenicity, and indirect effects of PCVs in under 15-year-olds. Another recent systematic review from 2014 focused only on otitis media in under 12 year-olds (10). These reviews reported PCVs to be effective in some studies but results remained partly inconclusive because data were not pooled. In the current systematic review, we pooled results for various patient-relevant outcomes, thoroughly examined heterogeneity, and set our results in context by providing examples for NNTs applicable to Germany.
Conclusions
This comprehensive systematic review and meta-analysis found significant risk reductions with PCVs for all-cause and vaccine-specific pneumonia, IPD, and AOM. We found no significant effect on all-cause mortality.
.
Key Messages.
Streptococcus pneumoniae (Pneumococcus) is a globally relevant pathogen against which pneumococcal conjugate vaccines have been studied in numerous randomized controlled trials.
The present systematic review is the first to assess randomized controlled trials on pneumococcal conjugate vaccines across different populations and settings and to summarize several patient-relevant outcomes.
Despite different populations and settings, the studies show relatively consistent results (low heterogeneity)
Pneumococcal conjugate vaccines effectively prevent diseases caused by the serotypes in the vaccine (relative risk reductions between 20% and 75%).
Pneumococcal conjugate vaccines are less effective in preventing non-serotype specific infectious diseases such as all-cause pneumonia (relative risk reductions between 5% and 55%), and not reliably effective in reducing the overall mortality rate (95% confidence interval of 12% relative risk reduction to 3% relative risk increase).
eFigure 1.
Study selection process
Acknowledgments
The authors thank Heike Raatz and Philipp Schütz for their help in the initiation of this project, and Kübra Özoglu for her excellent administrative support and assistance with literature management
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.World Health Organization. Pneumococcal disease. www.who.int/ith/diseases/pneumococcal/en/ (last accessed on 16 April 2015)
- 2.World Health Organization. Pneumococcal conjugate vaccine for childhood immunization-WHO position paper. Releve epidemiologique hebdomadaire/Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations. 2007;82:93–104. [PubMed] [Google Scholar]
- 3.O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization, UNICEF, World Bank. Geneva: World Health Organization; 2009. State of the world’s vaccines and immunization. [Google Scholar]
- 5.Frenck RW, Yeh S. The development of 13-valent pneumococcal conjugate vaccine and its possible use in adults. Expert Opin Biol Ther. 2012;12:63–77. doi: 10.1517/14712598.2012.636348. [DOI] [PubMed] [Google Scholar]
- 6.Lucero MG, Dulalia VE, Parreno RN, et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and pneumonia with consolidation on x-ray in children under two years of age. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004977. CD004977. [DOI] [PubMed] [Google Scholar]
- 7.Pavia M, Bianco A, Nobile CG, Marinelli P, Angelillo IF. Efficacy of pneumococcal vaccination in children younger than 24 months: a meta-analysis. Pediatrics. 2009;123:e1103–e1010. doi: 10.1542/peds.2008-3422. [DOI] [PubMed] [Google Scholar]
- 8.Davies EG, Riddington C, Lottenberg R, Dower N. Pneumococcal vaccines for sickle cell disease. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD003885.pub2. CD003885. [DOI] [PubMed] [Google Scholar]
- 9.Chang CC, Singleton RJ, Morris PS, Chang AB. Pneumococcal vaccines for children and adults with bronchiectasis. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD006316.pub2. CD006316. [DOI] [PubMed] [Google Scholar]
- 10.Fortanier AC, Venekamp RP, Boonacker CW, et al. Pneumococcal conjugate vaccines for preventing otitis media. Cochrane Database Syst Rev. 2014;4 doi: 10.1002/14651858.CD001480.pub4. CD001480. [DOI] [PubMed] [Google Scholar]
- 11.Conklin L, Loo JD, Kirk J, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type Invasive pneumococcal disease among young children. Pediatr Infect Dis J. 2014;33:109–118. doi: 10.1097/INF.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loo JD, Conklin L, Fleming-Dutra KE, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on prevention of pneumonia. Pediatr Infect Dis J. 2014;33:140–151. doi: 10.1097/INF.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schönberger K, Kirchgässner K, Riedel C, von Kries R. Effectiveness of 2+ 1 PCV7 vaccination schedules in children under 2 years: A meta-analysis of impact studies. Vaccine. 2013;31:5948–5952. doi: 10.1016/j.vaccine.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Chevallier B, Vesikari T, Brzostek J, et al. Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines. Pediatr Infect Dis. 2009;28:109–118. doi: 10.1097/INF.0b013e318199f62d. [DOI] [PubMed] [Google Scholar]
- 15.Oosterhuis-Kafeja F, Beutels P, Van Damme P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998-2006) Vaccine. 2007;25:2194–2212. doi: 10.1016/j.vaccine.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Thompson A, Gurtman A, Patterson S, et al. Safety of 13-valent pneumococcal conjugate vaccine in infants and children: meta-analysis of 13 clinical trials in 9 countries. Vaccine. 2013;31:5289–5295. doi: 10.1016/j.vaccine.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Wise RP, Iskander J, Pratt RD, et al. Postlicensure safety surveillance for 7-valent pneumococcal conjugate vaccine. JAMA. 2004;292:1702–1710. doi: 10.1001/jama.292.14.1702. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Pneumococcal vaccines WHO position paper 2012. Wkly Epidemio Rec. 2012;87:129–144. [PubMed] [Google Scholar]
- 19.Ewald H, Gloy V, Briehl M, et al. Pneumococcal conjugate vaccines for preventing infectious diseases and mortality: protocol for a systematic review of randomised controlled trials. PROSPERO. 2014 CRD42014009005 www.crd.york.ac.uk/PROSPERO_REBRANDING/display_record.asp?ID=CRD42014009005. [Google Scholar]
- 20.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 51.0 [updated March 2011] The Cochrane Collaboration. 2011 [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis. 2002;21:810–815. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Nachman S, Kim S, King J, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants with human immunodeficiency virus type 1 infection. Pediatrics. 2003;112:66–73. doi: 10.1542/peds.112.1.66. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362:355–361. doi: 10.1016/S0140-6736(03)14022-6. [DOI] [PubMed] [Google Scholar]
- 26.Bassler D, Briel M, Montori VM, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303:1180–1187. doi: 10.1001/jama.2010.310. [DOI] [PubMed] [Google Scholar]
- 27.Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 28.Dransfield MT, Nahm MH, Han MK, et al. Superior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:499–505. doi: 10.1164/rccm.200903-0488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson LA, Gurtman A, Rice K, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013;31:3585–3593. doi: 10.1016/j.vaccine.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Jackson LA, Gurtman A, van Cleeff M, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. 2013;31:3577–3584. doi: 10.1016/j.vaccine.2013.04.085. [DOI] [PubMed] [Google Scholar]
- 31.Macintyre CR, Ridda I, Gao Z, et al. A randomized clinical trial of the immunogenicity of 7-valent pneumococcal conjugate vaccine compared to 23-valent polysaccharide vaccine in frail, hospitalized elderly. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammitt LL, Ojal J, Bashraheil M, et al. Immunogenicity, impact on carriage and reactogenicity of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine in Kenyan children aged 1-4 years: a randomized controlled trial. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pomat WS, van den Biggelaar AH, Phuanukoonnon S, et al. Safety and immunogenicity of neonatal pneumococcal conjugate vaccination in Papua New Guinean children: a randomised controlled trial. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmu AA, Jokinen J, Borys D, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet. 2013;381:214–222. doi: 10.1016/S0140-6736(12)61854-6. [DOI] [PubMed] [Google Scholar]
- 35.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Klugman KP, Madhi SA, Huebner RE, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 37.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infec. 2014;20:45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 38.Barten G, Schutte H, Bals R, Pletz M, Rohde G. [Symposium: Pneumonia 2010—state of the art] Pneumologie (Stuttgart, Germany) 2011;65:223–228. doi: 10.1055/s-0030-1256275. [DOI] [PubMed] [Google Scholar]
- 39.Robert Koch-Institut. Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut: Begründungen zur allgemeinen Empfehlung der Impfungen gegen Pneumokokken und Meningokokken im Säuglings- und Kindesalter. Epidemiologisches Bulletin. 2006;31:255–270. [Google Scholar]
- 40.van der Linden M, Weiß S, Falkenhorst G, Siedler A, Imöhl M, von Kries R. Four years of universal pneumococcal conjugate infant vaccination in Germany: Impact on incidence of invasive pneumococcal disease and serotype distribution in children. Vaccine. 2012;30:5880–5885. doi: 10.1016/j.vaccine.2012.06.068. [DOI] [PubMed] [Google Scholar]
- e1.Liese JG, Silfverdal SA, Giaquinto C, et al. Incidence and clinical presentation of acute otitis media in children aged < 6 years in European medical practices. Epidemiol Infect. 2014;142:1778–1788. doi: 10.1017/S0950268813002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clinical infectious diseases. An official publication of the Infectious Diseases Society of America. 2000;30:100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- e3.Madhi SA, Petersen K, Madhi A, Wasas A, Klugman KP. Impact of human immunodeficiency virus type 1 on the disease spectrum of Streptococcus pneumoniae in South African children. Pediatr Infect Dis J. 2000;19:1141–1147. doi: 10.1097/00006454-200012000-00004. [DOI] [PubMed] [Google Scholar]
- e4.European Centre for Disease Prevention and Control Annual Epidemiological Report 2013. Reporting on 2011 surveillance data and 2012 epidemic intelligence data. Stockholm: ECDC. 2013 [Google Scholar]
- e5.Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- e6.Davis S, Feikin D, Johnson HL. The effect of Haemophilus influenzae type B and pneumococcal conjugate vaccines on childhood meningitis mortality: a systematic review. BMC Public Health. 2013 13;(Suppl 3) doi: 10.1186/1471-2458-13-S3-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Taylor S, Marchisio P, Vergison A, Harriague J, Hausdorff WP, Haggard M. Impact of pneumococcal conjugate vaccination on otitis media: a systematic review. Clin Infect Dis. 2012;54:1765–1773. doi: 10.1093/cid/cis292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Deloria Knoll M, Park DE, Johnson TS, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on immunogenicity. Pediatr Infect Dis J. 2014;33:119–129. doi: 10.1097/INF.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Fleming-Dutra KE, Conklin L, Loo JD, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage. Pediatr Infect Dis J. 2014;33:152–160. doi: 10.1097/INF.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Loo JD, Conklin L, Fleming-Dutra KE, et al. Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization. Pediatr Infect Dis J. 2014;33:161–171. doi: 10.1097/INF.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.O’Brien KL, Goldblatt D, Whitney CG. Why Do we need a systematic review of pneumococcal conjugate vaccine dosing schedules? Pediatr Infect Dis J. 2014;33:107–108. doi: 10.1097/INF.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Park DE, Johnson TS, Nonyane BAS, et al. The differential impact of coadministered vaccines, geographic region, vaccine product and other covariates on pneumococcal conjugate vaccine Immunogenicity. Pediatr Infect Dis J. 2014;33:130–139. doi: 10.1097/INF.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e13.Whitney CG, Goldblatt D, O’Brien KL. Dosing schedules for pneumococcal conjugate vaccine: Considerations for policy makers. Pediatr Infect Dis J. 2014;33:172–181. doi: 10.1097/INF.0000000000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e14.Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane database Syst Rev. 2013;1 doi: 10.1002/14651858.CD000422.pub3. CD000422. [DOI] [PMC free article] [PubMed] [Google Scholar]