Abstract
18F-fluorodeoxyglucose positron emission tomography with (FDG-PET) has a well-established role in the pre- and post-treatment staging of Hodgkin lymphoma (HL), however its use as a predictive therapeutic tool via responded-adapted therapy continues to evolve. There have been a multitude of retrospective and noncontrolled clinical studies showing that early (or interim) FDG-PET is highly prognostic in HL, particularly in the advanced-stage setting. Response-adapted treatment approaches in HL are attempting to diminish toxicity for low-risk patients by minimizing therapy, and conversely, intensify treatment for high-risk patients. Results from phase III noninferiority studies in early-stage HL with negative interim FDG-PET that randomized patients to chemotherapy alone versus combined modality therapy showed a continued small improvement in progression-free survival for patients who did not receive radiation. Preliminary reports of data escalating therapy for positive interim FDG-PET in early-stage HL and for de-escalation of therapy [i.e. bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone (BEACOPP)] for negative interim FDG-PET in advanced stage HL (i.e. deletion of bleomycin) have demonstrated improved outcomes. Maturation of these studies and continued follow up of all response-adapted studies are needed. Altogether, the treatment of HL remains an individualized clinical management choice for physicians and patients. Continued refinement and optimization of FDG-PET is needed, including within the context of targeted therapeutic agents. In addition, a number of new and novel techniques of functional imaging, including metabolic tumor volume and tumor proliferation, are being explored in order to enhance staging, characterization, prognostication and ultimately patient outcome.
Keywords: contrast-enhanced computerized tomography, 18F-fluorodeoxyglucose positron emission tomography, interim positron emission tomography, Hodgkin lymphoma, positron emission tomography, prognosis
Introduction
The majority of Hodgkin lymphoma (HL) patients achieve complete remission and cure with current treatment paradigms. However, a minority of patients will experience relapse and death [Canellos et al. 2014]. In addition, given the young age at diagnosis and overall high survival rates, serious acute and long-term treatment-related toxicities remain a concern including second malignancies, arterial disease, and negative impact on quality of life [Eichenauer et al. 2014; Yeh and Diller, 2012; Hodgson, 2011; Greaves et al. 2014; Khimani et al. 2013]. There remains an unmet need for predictive tools to help guide individualized treatment decisions for patients. This includes the identification of high-risk HL patients where more intensive therapy may be indicated, and conversely, the attenuation of treatment in lower risk patients in an attempt to decrease acute toxicity and late effects.
Functional imaging with 18F-fluorodeoxyglucose positron emission tomography with (FDG-PET) noncontrast computerized tomography (CT) has become a standard tool together with contrast-enhanced CT scan for the initial staging and re-assessment of HL [Evens and Kostakoglu, 2014; Kostakoglu and Evens, 2014]. FDG-PET scans have been shown to more accurately identify correct pretreatment stage when compared with contrast-enhanced CT (CECT), also causing upstaging to a more advanced stage [Hutchings et al. 2006a, Isasi et al. 2005]. The role of post-treatment FDG-PET has also been evaluated extensively to distinguish viable metabolically-active tumor from fibrotic or necrotic tissue in residual masses. However, a number of questions remain regarding the potential value of FDG-PET as a predictive tool in HL. This review focuses on the reproducibility and interpretation of FDG-PET, studies incorporating ‘early’ response-adapted FDG-PET, and the use of FDG-PET in the setting of relapsed or refractory HL. Other papers delineating the role of FDG-PET in the staging and post-treatment surveillance of HL patients has been reviewed elsewhere [Kostakoglu and Evens, 2014].
Interpretation and reproducibility of FDG-PET
The nonspecific nature of low-to-moderate grade residual uptake within a tumor mass during therapy limits the specificity of FDG-PET readings. The imaging subcommittee of the International Harmonization Project in Lymphoma in 2007 was the first initiative for standardization of FDG-PET interpretation following treatment [Cheson et al. 2007]. The resultant criteria stipulated that FDG-uptake greater than that of the mediastinal blood pool in residual masses greater than or equal to 2 cm was considered positive for residual lymphoma. Of note, these criteria were not recommended for application in interim FDG-PET interpretation and were based upon a retrospective study of 54 diffuse large B-cell lymphoma (DLBCL) patients, which were not validated in HL patients [Juweid et al. 2005]. Subsequent efforts to develop a more specific interpretation method has yielded the Deauville 5-point scale reading system (5PS) (Table 1).
Table 1.
Deauville 5-point scale criteria for evaluation of interim positron emission tomography.
| Score | Criteria |
|---|---|
| 1 | No uptake |
| 2 | Uptake < mediastinum |
| 3 | Uptake > mediastinum but < liver |
| 4 | Moderately increased uptake > liver |
| 5 | Markedly increased uptake > liver |
Recommended scoring of positive interim FDG-PET for early-stage Hodgkin lymphoma (score 3–5); recommended scoring for positive FDG-PET for advanced-stage Hodgkin lymphoma (score 4–5).
The Deauville 5PS allows for more accurate measurement of response by using a categorical scoring system with a continuous variable. It also allows for different thresholds for positive and negative tests to assess chemotherapy sensitivity versus response to chemotherapy. Using liver uptake with relatively high background uptake and higher positive predictive value (PPV) is preferable for therapy intensification, which will minimize overtreatment, toxicity and decrease the rate of false positives. It is important to highlight that a higher negative predictive value (NPV) can be achieved using mediastinal blood pool uptake, which may be useful when decreasing therapy intensity to minimize treatment [Meignan et al. 2009, 2010, 2012]. Furthermore, the patient acts as their own control in this method by comparing to a reference organ with generally consistent metabolic activity, reducing inter-reader and inter-device inconsistencies [Barrington et al. 2010].
The improved prognostic value of FDG-PET was confirmed in a study showing that NPV was high with all of the criteria, but using a high threshold for positive interim FDG-PET led to increased PPV (Table 2) [LeRoux et al. 2011]. Using the Deauville 5PS increased the PPV from 19% to 45%. Interim FDG-PET correlated strongest with progression-free survival (PFS) using 5PS criteria (p < 0.0001). An international multicenter retrospective cohort study of 260 advanced-stage HL patients imaged after two of six intended cycles (PET-2) of doxorubicin, bleomycin, vinblastine and dacarabazine (ABVD) confirmed the reproducibility of the Deauville 5PS [Gallamini et al. 2014]. No treatment changes were made based upon PET-2 results. Sensitivity, specificity, NPV and PPV were 73%, 94%, 94% and 73%, respectively. The 3 year failure-free survival was 28% for PET-2-positive patients and 95% for PET-2-negative patients (p < 0.0001) with a mean follow-up of 27 months. There was high binary concordance between paired reviewers (Cohen κ = 0.84). NPV and PPV of FDG-PET in HL may be disease- and treatment-specific and these results should not be applied to diseases or therapies other than HL and ABVD.
Table 2.
Observational studies of interim PET (nonadaptive) in Hodgkin lymphoma.
| Study | n | Prospective | Stages | Chemo regimen | Number of cycles before interim PET | % PET+ | PPV (%) | NPV (%) | % 2-y PFS |
% 2-y PFS |
Med F/U (mos) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PET+ | PET - | ||||||||||
| Hutchingset al. [2005] | 85 | No | I–IV | ABVD | 2–3 | 15 | 62 | 94 | 46 | 97 | 40 |
| 33% III-IV | |||||||||||
| Hutchingset al. [2006b] | 77 | Yes | I–IV | ABVD | 2 | 21 | 69 | 95 | 0 | 96 | 23 |
| 36% III-IV | |||||||||||
| Gallaminiet al. [2007] | 260 | Yes | IIA, IIB-IV | ABVD | 2 | 19 | 86 | 95 | 13 | 95 | 26 |
| 47% III-IV | |||||||||||
| Zinzani et al. [2012] | 304 | No | I–IV | ABVD | 2 | 17 | 92 | 72 | 13 | 95 | 45 |
| 51.5% III-IV | |||||||||||
| Cerci et al. [2010] | 104 | Yes | I–IV | ABVD | 2 | 29 | 53 | 92 | 3-y PFS: 53 | 3-y PFS: 90 | 36 |
| 59% III–IV | |||||||||||
| Sher et al. [2009] | 46 | No | I–II | ABVD | – | 43 | 15 | 96 | 85 | 96 | – |
| Kostakoglu et al. [2012] | 88 | Yes | IIB, 20% IIB | AVG | 2 | 27 | 46 | 84 | 50 | 89 | 39 |
| Gallaminiet al. [2011] | 165 | No | IIB–IVB or IIA w/ risk factors | ABVD→ | 2 | 17 | 39 | 90.5 | 2-y FFS: 65 | 2-y FFS: 92 | 34 |
| 53% III–IV | PET+ escBEACOPPx 4 then BEACOPPx 4 | ||||||||||
| PET-: ABVD |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; AVG, doxorubicin, vinblastine, gemcitabine; BEACOPP, bleomycin, etoposide, doxorubicin, vincristine, prednisone, procarabzine; esc, escalated; PET, positron emission tomography; PPV, positive predictive value; NPV, negative predictive value; 2-y PFS, 2-year progression-free survival; Med F/U, median follow up.
Early-stage HL
Current treatment recommendations for early-stage HL patients with favorable risk involves combined modality treatment, usually consisting of 2–3 cycles of ABVD followed by 20–30 Gy of involved field radiotherapy (IFRT) or involved node RT (INRT). For unfavorable risk profile early-stage HL, recommended therapy is four cycles of chemotherapy followed by 30 Gy of IFRT/INRT. Chemotherapy alone given for four to six cycles is an alternative treatment option in early-stage HL [Hay and Meyer, 2014]. Four published randomized clinical trials compared combined modality therapy (CMT) versus chemotherapy alone for treatment of adult early-stage HL [Laskar et al. 2004; Meyer et al. 2005; Nachman et al. 2002; Straus et al. 2004]. Disease control, measured by freedom from progression, freedom from treatment failure or event-free survival (EFS), was better with CMT in each study. Among the aforementioned studies, the absolute improvements were 3–7% in acute failure rates without radiation. Overall survival (OS) was similar in each study, with the NCIC-CTG/ECOG HD.6 study showing superior OS for chemotherapy alone at 12 years, with increased late toxicity and events in the CMT arm [Meyer et al. 2012]. Collectively, CMT has shown improvement in PFS versus chemotherapy alone in early-stage HL patients, but it has not translated to improvement in OS. The choice of initial therapy in early-stage HL continues to be strongly debated [Meyer and Hoppe, 2012]. In an attempt to have a predictive tool that identifies low-risk patients whereby radiation may be withheld, interim response-adapated FDG-PET has been studied extensively.
Observational/prospective studies (nonresponse adapted)
There have been comparatively less early-stage response adapted analyses compared with advanced stage HL (Table 2). Initial reports of interim FDG-PET for early-stage HL demonstrated a consistently high NPV and low-moderate PPV in relation to treatment outcome. There may be a high number of false-positive FDG-PET scans with the high incidence of inflammatory processes in HL.
A retrospective analysis of 85 HL patients with interim PET after two or three cycles of ABVD showed that FDG-PET had less robust progostication for early-stage versus advanced-stage HL patients [Hutchings et al. 2005]. Among 57 early stage patients, interim FDG-PET was prognostic for 2 year PFS (p = 0.003), however only two of seven interim PET-positive early-stage HL patients relapsed. Ann Arbor stage retained strong prognostic significance on multivariate analysis with interim FDG-PET included as a covariate. A later prospective study of patients with early- and advanced-stage HL showed extranodal disease and positive interim-PET as predictive of outcome [Hutchings et al. 2006b]. No early-stage HL patients with negative FDG-PET progressed (0/26) and only 1 of the 5 with PET-2-positive had progression. Sher and colleagues reported 2-year failure-free survival (FFS) rates of 92% and 69% for patients undergoing consolidation RT versus no RT for residual FDG-PET avidity after ABVD treatment [Sher et al. 2009]. In addition, a retrospective study in nonbulky limited-stage HL reported no difference in PFS for interim PET-positive and PET-negative patients treated with standard therapy [Barnes et al. 2011]. Notably, this analysis included early-stage HL patients treated without radiation consolidation (i.e. chemotherapy alone). These latter results from the Barnes et al. [2011] study highlight the overall poor PPV of interim PET in early-stage HL.
Efficacy of therapy is also a factor that can impact the predictive value of FDG-PET. A total of 88 patients with early-stage nonbulky HL were prospectively studied after treatment with a nonstandard regimen of doxorubicin, vinblastine and gemcitabine (AVG). Two-year PFS rates were 88% and 54% for PET-2-negative and PET-2-positive patients, respectively (p = 0.0009) [Straus et al. 2011]. The NPV of 86% was inferior to previously published early-stage HL data, which is likely due in part to the lower CR rate achieved with AVG (81%) compared with ABVD (94%).
Most interim FDG-PET prognostic analyses in early-stage HL have been in nonbulky patients. A recent analysis examined 121 consecutive early-stage HL patients with 30% patients having bulky disease (89% of which was mediastinal) [Pophali et al. 2014]. Interim FDG-PET was negative in 83% of bulky patients versus 95% of non-bulky (p = 0.17). Interim FDG-PET was prognostic for PFS and OS and it appeared to predict survival in bulky disease (PET-negative versus PET-positive: 5-year OS, 90% versus 75%, p = 0.012; 5-year PFS, 95% versus 25%, p < 0.01).
Phase II FDG-PET response-adapted studies
There have been a paucity of phase II studies reported utilizing response-adapted interim FDG-PET for early-stage HL. As previously noted, LeRoux and colleagues studied early- and advanced-stage HL patients treated with a response-adapted strategy PET-4 after ABVDx 4 [LeRoux et al. 2011]. The NPV and PPV with PET-4 for 2-year PFS were 95% and 16%, respectively (p < 0.0001). There are several ongoing phase II response-adapted studies ongoing for early-stage HL including in patients with bulky disease (Table 3).
Table 3.
Prospective noncontrolled response-adapted studies in Hodgkin lymphoma.
| Study | n | Stages | Chemo regimen | Number of cycles before interim PET | % PET+ | PPV (%) | NPV (%) | % 2-y PFS |
% 2-y PFS |
Med F/U (mos) |
|---|---|---|---|---|---|---|---|---|---|---|
| PET+ | PET - | |||||||||
| LeRoux et al. [2011] | 90 | I-IV | ABVD | 4 | 34 | 16 | 95 | NR | NR | 49 |
| 50% III-IV | ||||||||||
| CALGB 50604 | 160 | I/IIA-B | ABVDx 2→PET-2 | 2 | Accrual completed- results are pending | |||||
| non-bulky | PET – ABVDx 2 | |||||||||
| PET+ escBEACOPPx 2 +30 Gy IFRT | ||||||||||
| CALGB 50801 | 53 of 123 | I/IIA-B | ABVDx 2→PET-2 | 2 | Continuing accrual- results are pending | |||||
| bulky | PET- ABVDx 4 | |||||||||
| PET+ escBEACOPPx 4 +30 Gy IFRT | ||||||||||
| Avigdor et al. [2010] | 45 | IIB-IVB | escBEACOPP2, ABVD | 2 | 29 | 45 | 87 | 4-y PFS: 53% | 4-y PFS: 87% | 48 |
| 93% III-IV | ||||||||||
| Dann et al. [2012] | 108 | IIB-IV | escBEACOPP x 2, ABVD | 2 | 29 | 17 | 93 | 4-y PFS: 87% | 4-y PFS: 87% | 89 |
| 93% III-IV | ||||||||||
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; esc, escalated; PPV, positive predictive value; NPV, negative predictive value; PET, positron emission tomography;
2-year PFS, 2-year progression-free survival; Med F/U, median follow up.
Phase III response-adapted trials
A primary hypothesis of randomized studies in early-stage HL have been that acute disease control rates would be similar (noninferior) among patients with negative interim FDG-PET who receive radiotherapy versus no radiotherapy following chemotherapy. The H10F and H10U studies, led by the EORTC, randomized favorable and unfavorable early-stage HL patients (using EORTC definitions) to PET-based (experimental) versus non-PET-based (control) treatment strategies in noninferiority trials (Figure 1) [Raemaekers et al. 2014]. Assuming 5-year PFS of 95% in the standard arm for the favorable subgroup and 90% for the unfavorable subgroup, the investigators allowed for a decrease of 10% (to 85% and 80%, respectively) in the experimental PET-based arms. FDG-PET negativity was a Deauville 5PS score of 1 or 2 and pre-planned interim futility analyses were performed.
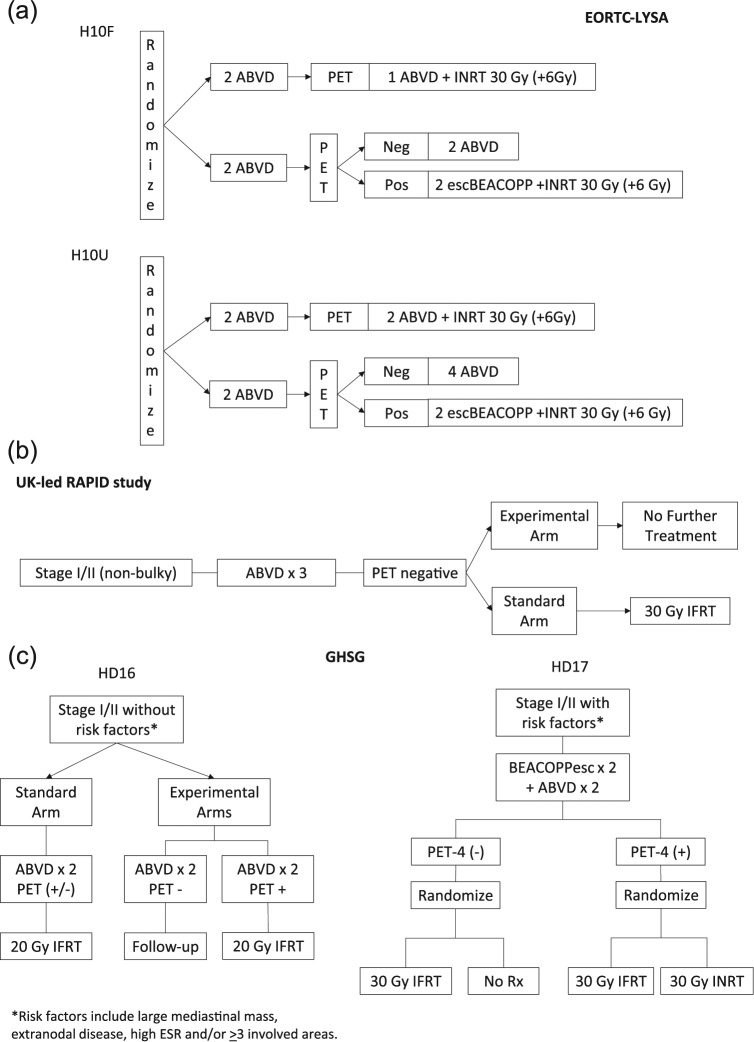
Figure 1.
Clinical trial designs of completed and ongoing phase III randomized studies of response-adapted therapy for adult early-stage HL. (A) EORTC/LYSA/FIL H10F study. *None of the following present: (a) large mediastinal mass; (b) age ⩾50 years; (c) high ESR; or (d) four or more areas. EORTC/LYSA/FIL H10U study. *Any of the following present: (a) large mediastinal mass; (b) age ⩾50 years; (c) high ESR; or (d) four or more areas. (B) UK-led RAPID study. All PET-3 + patients received a fourth cycle of ABVD followed by 30 Gy of involved field radiotherapy. (C) GHSG HD16 favorable trial. *None of the following present: (a) large mediastinal mass; (b) extranodal disease; (c) high ESR; or (d) three or more areas. GHSG HD17 unfavorable trial. *Any of the following present: (a) large mediastinal mass; (b) extranodal disease; (c) high ESR; or (d) three or more areas. High ESR for all of above defined as: >50 mm without B symptoms or ESR <30 mm with B symptoms.
Abbreviations: HL, Hodgkin lymphoma; EORTC, European Organisation for Research and Treatment of Cancer; esc, escalated; LYSA, Lymphoma Group and the Lymphoma Study Association; FIL, Fondazione Italiana Linfomi; pts, patients; GHSG, German Hodgkin Study Group; PET, positron emission tomography; ABVD, doxorubicin, bleomycin, vinblastine and dacarabazine; ESR, erythrocyte sedimentation rate.
In H10F, approximately 190 patients were randomized to each arm of the study. The PET-2-negative rate was 86%. At interim analysis, one event had occurred in the INRT arm versus nine events in the PET-based (no INRT) arm. The H10U study had approximately 260 patients randomized to each study arm with PET-2-negative rate of 75%. Seven events occurred in the INRT arm versus 16 events in the PET-based radiation-free arm. Statistical analyses for H10F and H10U showed that they would fail to reject the null hypotheses of inferiority of the experimental PET-based treatment arms and futility was declared for both studies (p = 0.017 and p = 0.026, respectively), despite the low absolute number of events. If accrual continued to originally planned numbers, it was unlikely that equivalence would be shown between the control and experimental arms. The study was amended to add INRT to all treatment arms and patient enrollment was increased in the FDG-PET arms to improve statistical power for the planned study objectives. A total of 1952 patients completed enrollment in June 2011. Complete results are pending.
Another objective of the H10F/U studies were to determine if intensification of therapy from ABVD therapy to escalated BEACOPP could improve outcomes for interim FDG-PET-2-positive patients [Raemaekers, 2015]. Preliminary data showed that a total of 361 patients had a positive interim FDG-PET-2, of which 188 received standard ABVD and 142 received escalated BEACOPP. Intention-to-treat analysis of ABVD versus escalated BEACOPP (BEACOPPesc) demonstrated decreased disease progression or relapse (19% versus 8%), death (9% versus 4%) and the first incidence of progression/relapse or death (21% versus 9%), all favoring BEACOPPesc. Further, 5-year PFS was 91% for BEACOPPesc + involved node RT (INRT) versus 77% for ABVD + INRT; 5-year OS was 96% for BEACOPPesc + INRT versus 89% ABVD + INRT, which was borderline significant (p = 0.06). Toxicity was higher in the BEACOPPesc arm, including grade 3 and 4 myelosuppression and infection. Final results of this dose escalation component of the study are eagerly awaited.
Results from the United Kingdom National Cancer Research Institute RAPID trial have recently been published (Table 4) [Radford et al. 2015]. This also was a phase III noninferiority randomized study that enrolled 602 patients with stage I/II nonbulky HL. All patients received three cycles of ABVD followed by FDG-PET (PET-3). Negative PET-3 was defined as Deauville 5PS 1–2. Patients with positive PET-3 went on to receive a fourth cycle of ABVD and IFRT, while PET-3-negative patients were randomized to IFRT versus no IFRT (Figure 1B). PET-3 was negative in 75% of patients. At a median follow-up of 60 months, 3 year PFS rates for PET-3-negative patients who received IFRT versus non-IFRT was 94.6% versus 90.8%, respectively. The 3-year absolute risk difference had 95% confidence intervals of 1.2 to −9.9%, with −9.9% limit exceeding the prespecified noninferiority boundary. On ‘per protocol’ analysis, 3-year PFS was 97% in the IFRT arm and 91% for non-IFRT (HR 2.36 (95% CI, 1.13–4.95), p = 0.02). On intent to treat, 3-year PFS was 95% in the IFRT arm and 91% for non-IFRT (HR 1.57 (95% CI, 0.84–2.97), p = 0.16). Altogether, these data suggest noninferiority was not present for 3-year PFS. Overall survival for 3 years was 97.1% in IFRT arm and 99% in the non-IFRT arm, which was nonsignificant. Three-year PFS and OS rates for patients with positive PET-3 were 87.6% and 94%, respectively.
Table 4.
Randomized phase III response-adapted studies in adult early-stage (I–II) Hodgkin lymphoma.
| Trial* | Patient group | Enrollment | Results |
|---|---|---|---|
| EORTC/LYSA/FIL H10F | Favorable Group | 761 patients: 381 patients PET-negative | 1-year PFS 100% (standard arm) and 95% (experimental arm); estimated HR 9.36 (79.6% CI 2.45–35.73) |
| EORTC/LYSA/FIL H10U | Unfavorable/Intermediate Group | 1191 patients: 519 patients PET-negative | 1-year PFS 97% (standard arm) and 94.7% (experimental arm); estimated HR 2.42 (80.4% CI 1.35–4.36) |
| EORTC/LYSA/FIL H10F/U | Favorable and Intermediate groups | >400 patients PET-positive (BEACOPPesc versus ABVD) | 5-year PFS 91% versus 77% (p = 0.002) and 5-year OS 96% versus 89% (p = 0.06) |
| UK NCRI RAPID | Favorable and unfavorable/intermediate groups combined (non-bulky) | 602 patients | 3-year PFS for no RT versus IFRT in PET neg pts: 91% versus 95% by ITT (p = 0.23) and 91% versus 97% by protocol analysis (p = .03) |
| 3-year PFS for PET pos: 85% | |||
| GHSG HD16 [ClinicalTrials.gov identifier: NCT01356680] | Favorable group | >700 patients of planned 1100 | Results pending |
| GHSG HD17 [ClinicalTrials.gov identifier: NCT00736320] | Unfavorable/Intermediate group | >300 patients of planned 1100 | Results pending |
Treatment schemas in Figure 1.
Abbreviations: EORTC, European Organization for Research and Treatment of Cancer; LYSA, Lymphoma Study Association; FIL, Fondazione Italiana Linfomi; UK NCRI, United Kingdom National Cancer Research Institute; GHSG, German Hodgkin Study Group; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; ABVD, doxorubicin, bleomycin, vinblastine and dacarabazine.
How do we reconcile these ‘negative’ study outcomes given the preliminary data showing that interim FDG-PET in HL was prognostic for favorable outcomes for patients with a negative early scan? This lies in part in the difference between ‘prognostic’ and ‘predictive’ factors. A prognostic factor is a clinical or biologic characteristic that is objectively measurable and provides information on the likely outcome of cancer/disease in an untreated person to define the effects of patient, tumor, or imaging characteristics on patient outcome. This may be a blood test [e.g. lactate dehydrogenase (LDH)], set of combined clinical factors [e.g. international prognostic score (IPS)], or an imaging result (e.g. FDG-PET). On the other hand, a predictive factor is a clinical or biologic characteristic that provides information on the likely benefit from treatment to define the effect of treatment on the tumor. In other words, prognostic factors must be tested and proven to be truly predictive of patient outcome through modification in therapy.
Phase III clinical studies ongoing
HD16 and HD17 are GHSG-led noninferiority trials examining response-adapted therapy of favorable and unfavorable HL, respectively (Figure 2). Similar to the EORTC H10 study, these studies are randomizing patients to non-PET based standard therapy versus FDG-PET response-adapted therapeutic withholding radiotherapy for patients with negative interim FDG-PET. HD17 also utilizes escalated BEACOPP as a component of therapy. The non-inferiority margins were set at 5% in these trials.
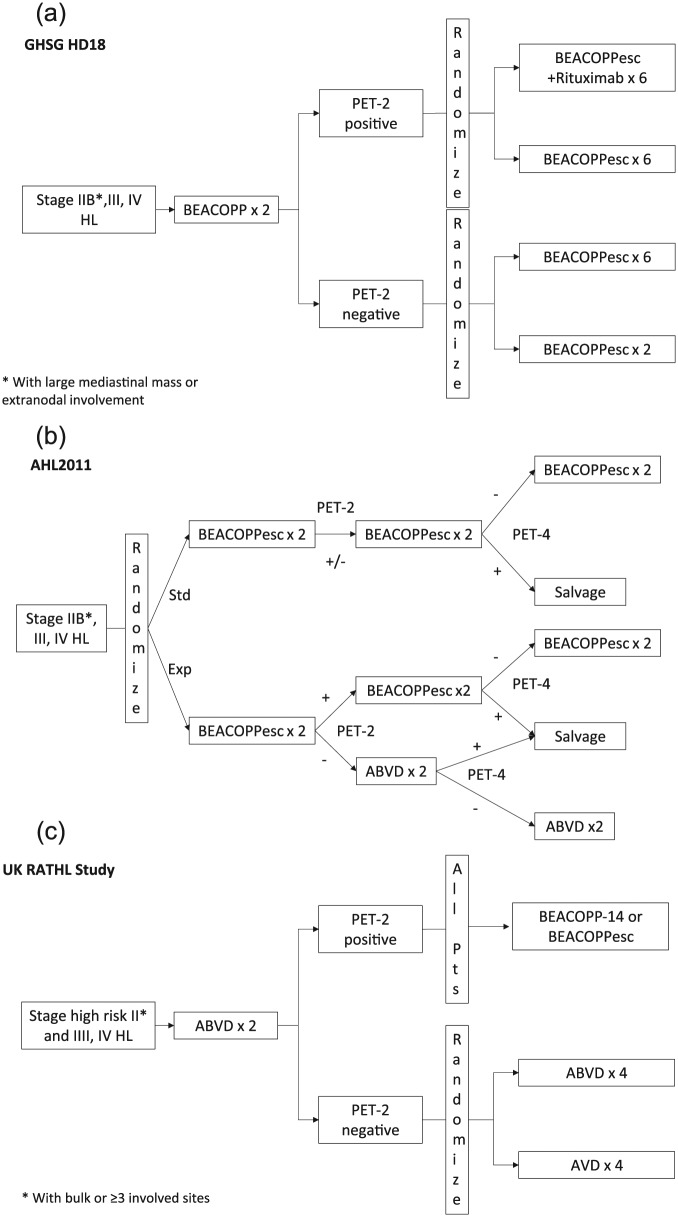
Figure 2.
Clinical trial designs of completed and ongoing phase III randomized studies of response-adapted therapy for adult advanced-stage HL. (A) GHSG HD18 includes patients with ‘high-risk’ stage II (*either of the following: large mediastinal mass or extranodal involvement) and stage III and IV disease. (B) The AHL2011 study includes patients with stage IIB and stage III and IV disease. (C) The Response Adapted Therapeutic Hodgkin Lymphoma (RATHL) study included patients with high-risk stage II disease (*with bulk or ⩾3 involved sites) and stage III and IV disease. Abbreviations: HL, Hodgkin lymphoma; Std, standard; Exp, experimental; esc, escalated; GHSG, German Hodgkin Study Group.
Advanced stage HL
Standard treatment for advanced-stage HL typically involves chemotherapy alone for six to eight cycles of therapy. The most common chemotherapy regimen recommended remains ABVD, however some authorities advocate utilization of the more intensive regimen, BEACOPP. The study of interim FDG-PET in advanced-stage HL has focused more on the identification of high-risk patients whereby therapy may be altered or intensified in order to improve outcomes.
Observational/prospective studies (nonresponse adapated)
The sensitivity and specificity of interim FDG-PET appear to be better for advanced-stage compared with early-stage HL (Table 2) [Cerci et al. 2010; Gallamini et al. 2006, 2007; Hutchings et al. 2005, 2006b; Terasawa et al. 2009; Zinzani et al. 2006, 2012]. A positive interim FDG-PET in advanced-stage HL has often been defined as a Deauville score of 4 or 5. Using this criteria with a higher cutpoint, the PPV will be improved (i.e. fewer false positives) so that a response-adapted strategy may identify high-risk patients with positive interim FDG-PET who may benefit from escalated treatment.
In a widely cited 2007 study, Gallamini and colleagues reported on the prognostic value of interim FDG-PET for newly-diagnosed advanced-stage HL. Among 260 patients, 190 had advanced stage disease; patients had FDG-PET after two cycles of ABVD (PET-2) [Gallamini et al. 2007] with or without IFRT following chemotherapy (Table 2). The 2 year PFS for PET-2-positive patients was 13% versus 95% for PET-2-negative patients. IPS and several factors were prognostic in univariate analysis, however interim FDG-PET was the dominant prognostic factor on multivariate analysis. In addition, a meta-analysis of 13 studies that included 360 untreated advanced stage HL patients showed that FDG-PET had an overall sensitivity of 81% and specificity of 97%. This analysis was limited by the inclusion of few high-risk patients (IPS 4–7) in the included trials [Terasawa et al. 2009].
A more recent retrospective study of 304 newly-diagnosed ABVD-treated HL patients examined the association of PET-2 with complete response (CR). A positive PET-2 was associated with continuous CR of 25%, while 92% of PET-2-negative patients achieved continuous CR at median of 31 months [Zinzani et al. 2012]. Another prospective study of 104 patients with HL showed a 3-year EFS of 55% for PET-2-positive patients versus 94% EFS for PET-2-negative patients [Cerci et al. 2010] (Table 2).
Phase II FDG-PET response-adapted studies
Investigators studied 160 patients with early-stage unfavorable or advanced-stage HL who were treated with ABVD and had therapy intensified to BEACOPP if PET-2 was positive; PET-2-negative patients remained on ABVD (Table 3) [Gallamini et al. 2011]. The 2-year FFS for PET-2-negative patients was 95%, while PET-2-positive patients had 2-year FFS of 62% with intensified therapy. PET-2 status was the only prognostic factor associated with FFS on multivariate analysis (p = 0.001). These findings suggest that early intensification with BEACOPP for PET-2-positive advanced-stage HL may improve outcomes, however there should be caution in interpreting these findings as this was not a randomized comparison.
Building upon this concept, several groups have performed studies integrating PET-adapted therapeutic strategies. Le Roux and colleagues examined a cohort of 54 patients with early unfavorable or advanced-stage HL treated following a PET-adapted strategy following four initial cycles of ABVD [LeRoux et al. 2011]. Among 31 patients with positive PET-4, 6 patients had treatment failure (19%), while 7 of 59 with a negative PET-4 (12%) had treatment failure. These results yielded a high NPV of 96%, but low PPV of 16%. The low PPV could be possibly explained by the combined criteria based on CT and PET results, incomparability of criteria used for interim FDG-PET interpretation, and relatively late timing for FDG-PET.
The Haifa study group has prospectively examined a cohort of 124 advanced-stage HL patients using interim FDG-PET (response-adapted) and IPS score for an adaptive treatment strategy [Dann et al. 2007, 2012] (Table 3). Patients with IPS score 0–2 (low risk) and 3–7 (high risk) were treated with two cycles of baseline or escalated BEACOPP, respectively. An interim Gallium-67 or FDG-PET was done to determine subsequent therapy, with continuation of escalated BEACOPP if imaging was positive or de-escalation to baseline BEACOPP with negative imaging. Ten-year PFS and OS were 87% and 88%, respectively, with median follow up of 89 months. For patients with positive interim FDG-PET, 10-year PFS was 83%; 10-year PFS was 93% for patients with negative interim FDG-PET. Avigdor and colleagues studied 45 patients with new diagnosis of HL stages IIB-IVB and IPS of at least 3 who were treated with two courses of escalated BEACOPP (Table 3) [Avigdor et al. 2010]. Interim FDG-PET and contrast-enhanced CT were used to determine response and treatment arms. By IHP criteria, patients in CR or PR had de-escalation with ABVD for four cycles, while patients with less than a PR proceeded to autologous stem cell transplant (SCT). A total of 44 patients were in CR or PR and 70% had a negative PET-2, while 30% had a positive PET-2. Patients with negative PET-2 and positive PET-2 had CR rates of 97% and 69%, respectively, which yielded a PPV of 45% and NPV of 87%. Four-year PFS was 87% for PET-2-negative patients and 53% for PET-2-positive patients, which was statistically different (p < 0.01).
A retrospective study reported at ASH 2014 examined the prognostic role of baseline FDG-PET with interim PET-2 with ABVD in untreated advanced HL [Cimino et al. 2014]. Among 162 patients analyzed with both PET and CECT, 57 patients were found to have extranodal (EN) disease. Baseline FDG-PET identified 27 EN sites of involvement missed by CECT, whereas CECT picked up 5 EN sites missed by PET-0 (25 EN sites were seen on both modalities). Univariate and multivariate analysis showed EN disease and positive PET-2 as the only significant variables on EFS with HRs of 3.9 [95% confidence interval (CI) 1.62–9.36, p = 0.002] and 2.9 (95% CI 1.82–4.52, p < 0.00001), respectively. Patients with both EN disease and positive PET-2 had 3-year EFS of 0%.
The US Intergroup S0816 is a phase II study that has reported preliminary results of 371 advanced-stage HL patients treated with ABVD as noted in Table 5 [Press et al. 2013]. Patients with interim negative PET-2 continued with four further cycles of ABVD, while PET-2-positive patients had therapy intensified to escalated BEACOPP for six cycles. PET-2 was negative in 82% and 2-year PFS was 78% for these patients. The 2-year PFS was 61% for PET-2-positive patients, which appeared improved compared with historical controls. Notably, HIV positive patients were included, but had therapy changed to baseline BEACOPP with positive PET-2. Of the 13 HIV positive patients, 11 were PET-2-negative. Twelve out of 13 patients were progression-free and all 13 remained alive. In addition, the Children’s Oncology Group (COG) has completed accrual to a phase II study with similar design to S0816, but utilizing ABVE-PC as the chemotherapy backbone, CT-defined interim analyses, and with the assignment of patients to one of two consolidation regimens based on response to induction therapy [ClinicalTrials.gov identifier: NCT01026220]. Patients with CT-defined rapid early response (RER) received two further cycles of ABVE-PC, and slow early responders had therapy intensified to ifosfamide and vinorelbine. An additional endpoint of this study is the investigation of FDG-PET after one cycle of induction ABVE-PC to examine comparability with RER. Results are awaited from this study.
Table 5.
Prospective response-adapted studies in advanced stage (III/IV) HL.
| Trial | Phase | Enrollment | Treatment | Outcomes |
|---|---|---|---|---|
| SWOG0816 | II | 371 | ABVDx 2→ PET-2 | 18% PET-2 pos |
| PET neg: ABVDx 4 (arm 1) | Arm 1 CR 96%, ORR 100%; | |||
| PET pos: escBEACOPPx 6 (arm 2) | Arm 2 CR 49%, ORR 85%; | |||
| HIV-neg: 2-year PFS 76%, 2-year OS: 95% | ||||
| COG AHOD0831 | II | 165 | ABVE-PCx 2→ PET-2 | Results not available |
| PETneg: ABVDx 2 (arm 1) | ||||
| PETpos: IV*x 2→ABVE-PCx 2 (arm 2) | ||||
| H2 study | II | 180 | IPS 0–2: ABVDx 2 →PET-2 | PET-2 pos: 15% (IPS 0–2: 12%, IPS 3–7: 20%) |
| IPS 3–7: escBEACOPPx 2→PET-2 | IPS 3–7: 80% pts had therapy de-escalated | |||
| PET-2 neg: ABVDx4 | 3-year PFS: 85% | |||
| PET-2 pos: escBEACOPP x4 | ||||
| GITIL HD0607 | III | 627 | ABVDx 2 → PET-2 | 18% PET-2 pos |
| PETneg: ABVDx 4, randomize RT versus no RT | 2nd interim analysis: 2-year PFS: 81% | |||
| PETpos: randomize escBEACOPP versus BEACOPP+/-rituximab | 2-year PFS PET-2-negative: 85% | |||
| 2-year PFS PET-2-positive: 61% | ||||
| HD0801 IIL | III | 520 | ABVDx 2 → PET-2 | Post-IGEV PET neg: 58%, Post-IGEV PET pos: 42% |
| PETneg: ABVDx4, randomize RT versus no RT | IGEV 2-year relapse free survival: 89% | |||
| PETpos: IGEV salvage | PET-2 neg: 2-year PFS: 78%, 2-year OS: 99% | |||
| Post-IGEV PET: neg: Auto SCT | PET-2 pos: 2-year PFS: 64%, 2-year OS: 86% | |||
| pos:tandem AutoSCT or Auto/Allo | ||||
| RATHL | III | 1412 | ABVDx 2→ PET-2 | 3-year PFS and OS: ABVD 85% and 97%, respectively versus AVD 84% and 98%, respectivelyPET-2 pos: 3-year PFS and OS 68% and 85% |
| PETneg: randomize ABVDx 4 versus AVDx 4 | ||||
| PETpos: escBEACOPP or BEACOPP14 | ||||
| GHSG HD18 | III | 1758 | EscBEACOPPx 2→PET-2 | Results pending |
| PET-2 neg: escBEACOPPx2 versus escBEACOPP x6 | ||||
| PET-2 pos: escBEACOPP+rituximab vs escBEACOPPx 6 | ||||
| LYSA AHL2011 | III | 810 | EscBEACOPPx 6 (Arm1) versus EscBEACOPPx 2→ PET-2 (Arm2) | Results pending |
| PET-2 neg: ABVDx 4 | ||||
| PET-2 pos: EscBEACOPPx 4 |
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; AVD, doxorubicin, vinblastine, dacarbazine; ABVE-PC: doxorubicin, bleomycin, vincristine, etoposide, prednisone, cyclophosphamide; IV, ifosfamide, vinorelbine; BEACOPP, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone; BEACOPP-14, 14-day cycle; esc, escalated; IGEV, ifosfamide, gemcitabine, etoposide, vinorelbine, prednisolone; PET, positron emission tomography; PETpos, positive PET scan; PETneg, negative PET scan; RT, radiotherapy;
ORR, overall response rate; CR, complete response; PFS, progression-free survival; OS, overall survival; IPS, international prognostic score; SWOG, Southwestern Oncology Group; COG, Children Oncology Group; UK NCRI, United Kingdom National Cancer Research Institute; RATHL, response adapted therapy for Hodgkin lymphoma; GITIL, Gruppo Italiano Terapie Innovative nei Linfomi; GHSG, German Hodgkin Study Group; LYSA, The Lymphoma Study Association.
The Italian GITIL0607 study has registered 730 patients treated with ABVD and subsequent response-adapted therapy (Table 5) [Gallamini et al. 2013]. A total of 82% of patients had negative PET-2 and proceeded to six total cycles of ABVD; PET-2-positive patients (18%) were randomized to escalated-BEACOPP for four cycles or standard BEACOPP for four cycles with or without rituximab. In the second interim analysis, 2-year PFS was 81% for all patients, with PET-2-negative and PET-2-positive PFS being 85% and 61%, respectively. Final results of this study are awaited. The HD0801 study enrolled 520 patients with advanced-stage HL in a response-adapted study with early transition to salvage therapy with SCT [Zinzani et al. 2013]. Patients with negative interim FDG-PET received six cycles of ABVD and were randomized to either radiation therapy to bulky mediastinal masses or no radiation therapy. Patients with positive interim FDG-PET received salvage therapy with IGEV (ifosfamide, gemcitabine, etoposide, vinorelbine and prednisolone); this was followed by autologous SCT with BEAM conditioning if FDG-PET post-IGEV was negative (58%). The 42% of patients with positive PET after IGEV had a tandem autologous SCT or autologous SCT followed by allogeneic SCT if a donor match was available. Two-year PFS and OS were 76% and 99% for interim PET-negative patients after two cycles of ABVD, while for PET-positive patients, the PFS and OS were 64% and 86%, respectively.
The Israeli H2 study has recruited 180 patients with advanced stage HL [Dann et al. 2013]. Patients with IPS 0–2 were treated initially with two cycles of ABVD, while patients in IPS 3–7 group were treated with two cycles of escalated BEACOPP. Negative PET-2 patients proceeded to four additional cycles of ABVD, while PET-2-positive patients received four cycles of escalated BEACOPP. After interim FDG-PET, 85% of patients were PET-2-negative and 15% positive. PET-2 was negative in 88% of the IPS 0–2 group and 80% of IPS 3–7 group. Furthermore, in the IPS 3–7 group, therapy was de-escalated in 89% of patients and 13% of the whole group had progression at 3 years. At a median follow up of 26 months, the 3-year PFS was 85%.
Phase III response-adapted trials
The ongoing GHSG HD18 trial is using an initial two initial cycles of escalated BEACOPP followed by FDG-PET assessment [Borchmann et al. 2012]. PET-2-negative patients were randomized to two versus six additional cycles of escalated BEACOPP, while PET-2-positive patients were randomized to six cycles of escalated BEACOPP with or without rituximab. Radiation therapy was used only for residual post-treatment FDG-avid disease. The LYSA AHL2011 study similarly uses upfront escalated BEACOPP for all patients [Casanovas et al. 2013]. Randomization occurs between the standard arm without response-adapted therapy and the experimental arm utilizes interim FDG-PET. In the standard arm, patients receive six cycles of escalated BEACOPP with negative PET-4. In the experimental arm, patients with negative PET-2 change to ABVD, while PET-2-positive patients continue with escalated BEACOPP (Table 5).
Preliminary results have been recently reported from a large randomized phase III study led by the UK NCRI in advanced-stage HL called the RATHL (Response-Adjusted Therapy for Hodgkin Lymphoma) study [Johnson et al. 2015]. Among 1214 enrolled advanced-stage HL patients, all patients received two cycles of ABVD followed by PET-2; 84% of patients had a negative PET-2 defined as Deauville scores of 4 or 5. PET-2-negative patients were randomized to continued ABVD or doxorubicin, vinblastine, and dacarbazine (AVD). PET-2-positive patients had their therapy intensified (i.e. BEACOPP-14). With a median follow-up of 32 months, PFS at 3 years appeared the same for ABVD 85% versus AVD 84% (OS 97% versus 98%, respectively). In addition, the ABVD arm had significantly more pulmonary toxicity than the AVD arm. Final analysis and publication of these data are eagerly awaited.
Newer imaging techniques
Combined CT and PET analyses
In an effort to improve positive predictive value of FDG-PET imaging, several studies combined PET and CT results to further separate favorable and unfavorable patient outcomes. In a study of 88 patients with stage I and II non-bulky HL incorporating IHP and Deauville 5PS criteria, the percentage of decrease in the sum of products of perpendicular diameters of masses after two cycles strongly correlated with 2-year PFS [Kostakoglu et al. 2012]. Analysis of PET-2 with CECT-2 data suggested improvement in prediction of 2-year PFS versus each test alone. For PET-2-positive patients, a negative CECT, which was defined as >65% decrease in size of a mass, decreased the false positive FDG-PET results and increased predictive value for PFS 27–35%. A limitation to this analysis was that some confidence intervals were not reliable due to small sample sizes. In the GHSG HD15 trial, in 739 advanced stage HL patients, CT alone did not allow further separation of patients in partial remission by risk of recurrence (p = 0.9). In the subgroup of the 54 PET-positive patients with a relative reduction of less than 40%, the risk of progression or relapse within the first year was 23% compared with 5% for patients with a larger reduction [Kobe et al. 2014]. These findings warrant further investigation of CECT in combination with PET.
Metabolically defined and quantifiable tumor volumes
Although FDG-PET has proved useful for therapy monitoring in HL patients, the false positivity due to post-therapy inflammatory processes have raised concerns about its effective use in interim PET-adapted strategies. In an effort to increase both the interpretation accuracy and reproducibility, various quantitative methods have been proposed [Casasnovas et al. 2011; Lin et al. 2007; Weber, 2007]. In HL, a maximum standardized uptake value (SUVmax) cut-off of 4.0 has reportedly provided the best joint sensitivity and specificity for the prediction of progression after two cycles of chemotherapy [Hutchings et al. 2006b]. It is important to note that these criteria have not been prospectively validated and also the SUV cutoff as a prognostic indicator may be different for defining response very early during therapy compared to later time points.
Other proposed PET parameters that include functional tumor volume parameters, for example, metabolically active tumor volume (MTV) and total lesion glycolysis (TLG) are currently evolving and have not yet been standardized, thus, not widely used. MTV incorporates the size-dependent thresholding to determine MTV on the basis of SUVmax obtained within a volume of interest that represents the tumor biology. Baseline quantitative PET parameters can be used as prognostic factors and may have better predictive value than conventional clinical prognostic factors, that is, in HL patients.
In an analysis of early-stage HL analysis, pretreatment MTV and SUVmax did not correlate with outcome, however change in MTV between interim and baseline studies was associated with median PFS (p = 0.01) as was SUVmax (p = 0.02) [Tseng et al. 2012]. In addition, a recent study examined the prognostic importance of baseline (pre-treatment) total MTV in a retrospective single-center study of 59 early-stage HL patients [Kanoun et al. 2014]. Baseline total MTV more accurately predicted outcome than tumor bulk and it was prognostic in multivariate analysis for PFS. Furthermore, the combination of MTV and ΔSUVmaxPET0-2 made it possible to identify three subsets of HL patients with different outcomes in terms of PFS and disease-specific survival (p < 0.0001). In these three groups the 4-year PFS rates were 92%, 49%, and 20% (p < 0.0001), respectively. In another retrospective study of early-stage HL patients that analyzed MTV in combination with a multitude of clinical prognostic factors, only high MTV (PFS, p = 0.008; OS, p = 0.007), older age, and B symptoms were significant independent prognostic factors for survival [Song et al. 2013].
New imaging biomarkers
New imaging biomarkers include measures of heterogeneity, which is emerging as an important factor in imaging analyses [Hatt et al. 2010]. Assessment of tumor proliferative activity may provide a critical tool for individualized treatment. The 3′-deoxy-3′-18 F-fluorothymidine (FLT) is the most extensively investigated functional imaging probe for measurement of cancer cell proliferative capacity [Bading and Shields, 2008]. The role of FLT-PET will depend in part in its ability to predict early response during treatment, rather than determining the extent of disease involvement at staging. The clinical utility of FLT as an early response surrogate to date has been demonstrated in preliminary clinical studies in non-HL [Bading and Shields, 2008].
There are a number of limitations to these early functional imaging studies. Methodologically, there are ongoing challenges associated with tumor segmentation algorithms. Consequently, currently used methods are often being used without the needed validation or optimization. Imaging parameters such as scanner resolution, reconstruction algorithms, filtering, tumor-to-background ratio, and image noise impacts the accuracy and precision of tumor delineation methods. This implies that technical PET parameters and the tumor delineation methods require standardization and calibration of each scanner for reproducible and accurate volume determinations. Collectively, despite promising preliminary results, the prognostic and predictive value of functional tumor volume remains to be further investigated with standardized, prospective, multicenter studies to determine the extent that these new imaging modalities may play in the management of HL.
Novel therapeutic agents
Brentuximab vedotin (BV) is an antibody drug conjugate with significant activity in patients with HL. The prognostic impact of interim FDG-PET (using Deauville 5PS > 3 as positive) was examined in a study using single-agent BV for relapsed/refractory HL [Kahraman et al. 2014]. After receiving a median of 3 doses BV, 67% were interim PET-positive. One year PFS rate for interim PET-negative patients was 100% compared with 38% for interim PET-positive patients (p = 0.033). FDG-PET was also studied in transplant eligible, relapsed/refractory HL using response-adapted treatment and salvage with single agent BV [Moskowitz et al. 2015]. In this phase II non-randomized study, 46 relapsed/refractory patients who had failed one prior doxorubicin-containing regimen were treated with two cycles of BV and then underwent FDG-PET scan, with Deauville 5PS 0–2 considered negative. PET-negative patients proceeded to autologous SCT, while PET-positive transitioned to salvage ICE (ifosfamide, carboplatin and etoposide) with repeat FDG-PET prior to SCT. Following single-agent BV, FDG-PET was negative in 27% of patients; altogether, 73% of patients became FDG-PET negative after salvage therapy, including ICE. Although these results are encouraging, more research is required to define the role of FDG-PET in determining optimal salvage therapy. The FDG-PET negative rate (using Deauville 5PS 1–3) for concurrent BV and AVD/ABVD chemotherapy in a phase I study of newly-diagnosed HL was 96% [Younes et al. 2013]. The prognostic impact of FDG-PET in the treatment of HL with novel therapeutic agents requires validation, although it represents an appealing strategy to predict efficacy. Finally, the prognostic impact, if any, of FDG-PET in the use and efficacy of PD-1 inhibitors in lymphoma continues to be explored [Bryan and Gordon, 2014].
Conclusions
FDG-PET has an established role in the pretreatment staging of HL, but its use as a predictive therapeutic tool with use as responded-adapted therapy continues to evolve. As reviewed, there have been multiple studies showing that interim FDG-PET is highly prognostic in HL, particularly in the advanced-stage setting. Prospective studies have evaluated the utility of interim FDG-PET for response-adapted treatment approaches in an attempt to diminish toxicity by minimizing therapy for low-risk patients and to potentially improve outcomes by intensifying treatment for high-risk HL patients. Initial studies in early-stage HL examining negative interim FDG-PET showed a continued small improvement in PFS for patients who received radiation therapy (i.e. similar to the pre-PET era). It is possible that late survival advantages may emerge with longer follow up (especially OS). Preliminary reports of data escalating therapy for positive interim FDG-PET in early-stage HL and for de-escalation of therapy for negative interim FDG-PET in advanced stage HL showed that outcomes were improved. Maturation and longer follow up of these data are needed. Furthermore, continued refinement and optimization of response-adapted therapy is needed, including in the context of targeted therapeutic agents. In addition, a number of new and novel techniques of functional imaging, such as metabolic tumor volume and tumor proliferation using FLT and integrated PET/MRI, are being explored in order to enhance staging, characterization, and prognostication in HL.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Michael Coyle, Tufts Medical Center in Boston, MA, USA.
Lale Kostakoglu, Icahn School of Medicine at Mount Sinai in New York, New York, USA.
Andrew M. Evens, Division of Hematology-Oncology, Director, Tufts Cancer Center, Tufts Medical Center, 800 Washington Street, Box #245, Boston, MA 02111, USA.
References
- Avigdor A., Bulvik S., Levi I., Dann E., Shemtov N., Perez-Avraham G., et al. (2010) Two cycles of escalated BEACOPP followed by four cycles of ABVD utilizing early-interim PET/CT scan is an effective regimen for advanced high-risk Hodgkin’s lymphoma. Ann Oncol 21: 126–132. [DOI] [PubMed] [Google Scholar]
- Bading J., Shields A. (2008) Imaging of cell proliferation: status and prospects. J Nucl Med 49(Suppl. 2): 64S–80S. [DOI] [PubMed] [Google Scholar]
- Barnes J., LaCasce A., Zukotynski K., Israel D., Feng Y., Neuberg D., et al. (2011) End-of-treatment but not interim PET scan predicts outcome in nonbulky limited-stage Hodgkin’s lymphoma. Ann Oncol 22: 910–915. [DOI] [PubMed] [Google Scholar]
- Barrington S., Qian W., Somer E., Franceschetto A., Bagni B., Brun E., et al. (2010) Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 37: 1824–1833. [DOI] [PubMed] [Google Scholar]
- Borchmann P., Eichenauer D., Engert A. (2012) State of the art in the treatment of Hodgkin lymphoma. Nat Rev Clin Oncol 9: 450–459. [DOI] [PubMed] [Google Scholar]
- Bryan L., Gordon L. (2014) Pidilizumab in the treatment of diffuse large B-cell lymphoma. Expert Opin Biol Ther 14: 1361–1368. [DOI] [PubMed] [Google Scholar]
- Canellos G., Rosenberg S., Friedberg J., Lister T., DeVita V. (2014) Treatment of Hodgkin lymphoma: a 50-year perspective. J Clin Oncol 32: 164–168. [DOI] [PubMed] [Google Scholar]
- Casasnovas R., Meignan M., Berriolo-Riedinger A., Bardet S., Julian A., Thieblemont C., et al. (2011) SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood 118: 37–43. [DOI] [PubMed] [Google Scholar]
- Casanovas R., Meignan M., Reman O., Gaillard I., Stamatoullas A. Brice, P.et al. (2013) AHL 2011: a LYSA randomized phase III study of a treatment driven by early PET response compared to a standard treatment in patients with Ann Arbor Stage III-IV or high-risk IIB Hodgkin lymphoma. J Clin Oncol 31: TPS815a. [Google Scholar]
- Cerci J., Pracchia L., Linardi C., Pitella F., Delbeke D., Izaki M., et al. (2010) 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J Nucl Med 51: 1337–1343. [DOI] [PubMed] [Google Scholar]
- Cheson B., Pfistner B., Juweid M., Gascoyne R., Specht L., Horning S., et al. (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25: 579–586. [DOI] [PubMed] [Google Scholar]
- Cimino G., Zaucha J., Cirillo S., Hutchings M., El-Galaly T., Borra A., et al. (2014) The complementary prognostic role of baseline and interim PET in predicting treatment outcome in advanced-stage Hodgkin lymphoma. ASH Annual Meeting Abstracts 124: 4405. [Google Scholar]
- Dann E., Bairey O., Bar-Shalom R., Izak M., Korenberg A., Akria L., et al. (2013) Tailored therapy in Hodgkin lymphoma, based on predefined risk factors and early interim PET/CT, Israeli H2 protocol: preliminary report on 317 patients. Haematologica 98(Suppl. 2): 37. [Google Scholar]
- Dann E., Bar-Shalom R., Tamir A., Haim N., Ben-Shachar M., Avivi I., et al. (2007) Risk-adapted BEACOPP regimen can reduce the cumulative dose of chemotherapy for standard and high-risk Hodgkin lymphoma with no impairment of outcome. Blood 109: 905–909. [DOI] [PubMed] [Google Scholar]
- Dann E., Blumenfeld Z., Bar-Shalom R., Avivi I., Ben-Shachar M., Goor O., et al. (2012) A 10-year experience with treatment of high and standard risk Hodgkin disease: six cycles of tailored BEACOPP, with interim scintigraphy, are effective and female fertility is preserved. Am J Hematol 87: 32–36. [DOI] [PubMed] [Google Scholar]
- Eichenauer D., Thielen I., Haverkamp H., Franklin J., Behringer K., Halbsguth T., et al. (2014) Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood 123: 1658–1664. [DOI] [PubMed] [Google Scholar]
- Evens A., Kostakoglu L. (2014) The role of FDG-PET in defining prognosis of Hodgkin lymphoma for early-stage disease. Blood 124: 3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallamini A., Barrington S., Biggi A., Chauvie S., Kostakoglu L., Gregianin M., et al. (2014) The predictive role of interim positron emission tomography on Hodgkin lymphoma treatment outcome is confirmed using the 5-point scale interpretation criteria. Haematologica 99: 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallamini A., Hutchings M., Rigacci L., Specht L., Merli F., Hansen M., et al. (2007) Early interim 2-[18F]fluoro-2-deoxy-D glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol 25: 3746–3752. [DOI] [PubMed] [Google Scholar]
- Gallamini A., Patti C., Viviani S., Rossi A., Fiore F., Di Raimondo F., et al. (2011) Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with interim-PET positive after two ABVD courses. Br J Haematol 152: 551–560. [DOI] [PubMed] [Google Scholar]
- Gallamini A., Rigacci L., Merli F., Nassi L., Bosi A., Capodanno I., et al. (2006) The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica 91: 475–481. [PubMed] [Google Scholar]
- Gallamini A., Rossi A., Patti C., Picardi M., Di Raimondo F., Cantonetti M., et al. (2013) Early treatment intensification in advanced-stage high-risk Hodgkin lymphoma patients, with a positive FDG-PET scan after two ABVD courses-second interim analysis of the GITIL/FIL HD0607 clinical trial. Haematologica 98: 3. [Google Scholar]
- Greaves P., Sarker S., Chowdhury K., Johnson R., Matthews J., Matthews R., et al. (2014) Fertility and sexual function in long-term survivors of haematological malignancy: using patient-reported outcome measures to assess a neglected area of need in the late effects clinic. Br J Haematol 164: 526–535. [DOI] [PubMed] [Google Scholar]
- Hatt M., Cheze le Rest C., Descourt P., Dekker A., De Ruysscher D., Oellers M., et al. (2010) Accurate automatic delineation of heterogeneous functional volumes in positron emission tomography for oncology applications. Int J Radiat Oncol Biol Phys 77: 301–308. [DOI] [PubMed] [Google Scholar]
- Hay A., Meyer R. (2014) Balancing risks and benefits of therapy for patients with favorable-risk limited-stage Hodgkin lymphoma: the role of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy alone. Hematol Oncol Clin North Am 28: 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson D. (2011) Late effects in the era of modern therapy for Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program 2011: 323–329. [DOI] [PubMed] [Google Scholar]
- Hutchings M., Loft A., Hansen M., Pedersen L., Berthelsen A., Keiding S., et al. (2006a) Positron emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica 91: 482–489. [PubMed] [Google Scholar]
- Hutchings M., Loft A., Hansen M., Pedersen L., Buhl T., Jurlander J., et al. (2006b) FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 107: 52–59. [DOI] [PubMed] [Google Scholar]
- Hutchings M., Mikhaeel N., Fields P., Nunan T., Timothy A. (2005) Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol 16: 1160–1168. [DOI] [PubMed] [Google Scholar]
- Isasi C., Lu P., Blaufox M. (2005) A meta-analysis of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography in the staging and restaging of patients with lymphoma. Cancer 104: 1066–1074. [DOI] [PubMed] [Google Scholar]
- Johnson P., Federico M., Fossa A., O’Doherty M., Roberts T., Stevens L., et al. (2015) Response-Adapted Therapy Based on Interim FDG-PET Scans in Advanced Hodgkin Lymphoma: First Analysis of the Safety of De-escalation in the International RATHL Study (CRUK/07/033). Presented at the 13th International Conference on Malignant Lymphoma, 17–20 June 2015, Lugano, Switzerland. [Google Scholar]
- Juweid M., Wiseman G., Vose J., Ritchie J., Menda Y., Wooldridge J., et al. (2005) Response assessment of aggressive non-Hodgkin’s lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol 23: 4652–4661. [DOI] [PubMed] [Google Scholar]
- Kahraman D., Theurich S., Rothe A., Kuhnert G., Sasse S., Scheid C., et al. (2014) 18-Fluorodeoxyglucose positron emission tomography/computed tomography for assessment of response to brentuximab vedotin treatment in relapsed and refractory Hodgkin lymphoma. Leuk Lymphoma 55: 811–816. [DOI] [PubMed] [Google Scholar]
- Kanoun S., Rossi C., Berriolo-Riedinger A., Dygai-Cochet I., Cochet A., Humbert O., et al. (2014) Baseline metabolic tumour volume is an independent prognostic factor in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging 41: 1735–1743. [DOI] [PubMed] [Google Scholar]
- Khimani N., Chen Y., Mauch P., Recklitis C., Diller L., Silver B., et al. (2013) Influence of new late effects on quality of life over time in Hodgkin lymphoma Survivors: a longitudinal survey study. Ann Oncol 24: 226–230. [DOI] [PubMed] [Google Scholar]
- Kobe C., Kuhnert G., Kahraman D., Haverkamp H., Eich H., Franke M., et al. (2014) Assessment of tumor size reduction improves outcome prediction of positron emission tomography/computed tomography after chemotherapy in advanced-stage hodgkin lymphoma. J Clin Oncol 32: 1776–1781. [DOI] [PubMed] [Google Scholar]
- Kostakoglu L., Evens A. (2014) FDG-PET imaging for Hodgkin lymphoma: current use and future applications. Clin Adv Hematol Oncol 12: 20–35. [PubMed] [Google Scholar]
- Kostakoglu L., Schöder H., Johnson J., Hall N., Schwartz L., Straus D., et al. (2012) Interim [(18)F]fluorodeoxyglucose positron emission tomography imaging in stage I-II non-bulky Hodgkin lymphoma: would using combined positron emission tomography and computed tomography criteria better predict response than each test alone? Leuk Lymphoma 53: 2143–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskar S., Gupta T., Vimal S., Muckaden M., Saikia T., Pai S., et al. (2004) Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine and dacarbazine chemotherapy: is there a need? J Clin Oncol 22: 62–68. [DOI] [PubMed] [Google Scholar]
- LeRoux P., Gastinne T., Le Gouill S., Nowak E., Bodet-Milin C., Querellou S., et al. (2011) Prognostic value of interim FDG PET/CT in Hodgkin’s lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging 38: 1064–1071. [DOI] [PubMed] [Google Scholar]
- Lin C., Itti E., Haioun C., Petegnief Y., Luciani A., Dupuis J., et al. (2007) Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med 48: 1626–1632. [DOI] [PubMed] [Google Scholar]
- Meignan M., Gallamini A., Haioun C., Polliack A. (2010) Report on the Second International Workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010 Leuk Lymphoma 51: 2171–2180. [DOI] [PubMed] [Google Scholar]
- Meignan M., Gallamini A., Itti E., Barrington S., Haioun C., Polliack A. (2012) Report on the Third International Workshop on interim positron emission tomography in lymphoma held in Menton, France, 26–27 September 2011 and Menton 2011. consensus. Leuk Lymphoma 53: 1876–1881. [DOI] [PubMed] [Google Scholar]
- Meignan M., Gallamini A., Meignan M., Gallamini A., Haioun C. (2009) Report on the First International Workshop on interim-PET-Scan in lymphoma Leuk Lymphoma 50: 1257–1260. [DOI] [PubMed] [Google Scholar]
- Meyer R., Gospodarowicz M., Connors J., Pearcey R., Bezjak A., Wells W., et al. (2005) Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol 23: 4634–4642. [DOI] [PubMed] [Google Scholar]
- Meyer R., Gospodarowicz M., Connors J., Pearcey R., Wells W., Winter J., et al. (2012) ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med 366: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R., Hoppe R. (2012) Point/counterpoint: early-stage Hodgkin lymphoma and the role of radiation therapy. Blood 120: 4488–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz A., Schöder J., Yaholem J., McCall S., Fox S., Gerecitano J., et al. (2015) PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosfamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol 16: 284–292. [DOI] [PubMed] [Google Scholar]
- Nachman J., Sposto R., Herzog P., Gilchrist G., Wolden S., Thomson J., et al. (2002) Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin’s disease who achieve a complete response to chemotherapy. J Clin Oncol 20: 3765–3771. [DOI] [PubMed] [Google Scholar]
- Pophali P., Rybicki L., Fenner K., Jagadeesh D., Dean R., Pohlman B., et al. (2014) Bulky disease does not adversely affect overall survival in early stage Hodgkin lymphoma: role of interim PET and possible omission of radiotherapy in select patients. ASH Annual Meeting Abstracts 124: 4428. [Google Scholar]
- Press O., LeBlanc M., Rimsza L., Schoder H., Friedberg J., Evens A., et al. (2013) A phase II trial of response-adapted therapy of stages III-IV hodgkin lymphoma using early interim fdg-pet imaging: U.S. intergroup s0816. Hematol Oncol 31: 137. [Google Scholar]
- Radford J., Illidge T., Counsell N., Hancock B., Pettengell R., Johnson P., et al. (2015) Results of a trial of PET-directed therapy for early-stage Hodgkin’s Lymphoma. N Engl J Med 372: 1598–1607. [DOI] [PubMed] [Google Scholar]
- Raemaekers J. (2015) Early FDG-PET Adapted Treatment Improves the Outcome of Early FDG-PET Positive Patients with Stages I/II Hodgkin Lymphoma (HL): Final Results of the Randomized Intergroup EORTC/LYSA/FIL H10 Trial. Presented at the 13th International Conference on Malignant Lymphoma, 17–20 June 2015, Lugano, Switzerland. [Google Scholar]
- Raemaekers J., André M., Federico M., Girinsky T., Oumedaly R., Brusamolino E., et al. (2014) Omitting radiotherapy in early positron emission tomography-negative stage I/II hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 32: 1188–1194. [DOI] [PubMed] [Google Scholar]
- Sher D., Mauch P., Van Den Abbeele A., LaCasce A., Czerminski J., Ng A. (2009) Prognostic significance of mid- and post-ABVD PET imaging in Hodgkin’s lymphoma: the importance of involved-field radiotherapy. Ann Oncol 20: 1848–1853. [DOI] [PubMed] [Google Scholar]
- Song M., Chung J., Lee J., Jeong S., Lee S., Hong J., et al. (2013) Metabolic tumor volume by positron emission tomography/computed tomography as a clinical parameter to determine therapeutic modality for early stage Hodgkin’s lymphoma. Cancer Sci 104: 1656–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D., Johnson J., LaCasce A., Bartlett N., Kostakoglu L., Hsi E., et al. (2011) Doxorubicin, vinblastine, and gemcitabine (CALGB 50203) for stage I/II nonbulky Hodgkin lymphoma: pretreatment prognostic factors and interim PET. Blood 117: 5314–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D., Portlock C., Qin J., Myers J., Zelenetz A., Moskowitz C., et al. (2004) Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood 104: 3483–3489. [DOI] [PubMed] [Google Scholar]
- Terasawa T., Lau J., Bardet S., Couturier O., Hotta T., Hutchings M., et al. (2009) Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin’s lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol 27: 1906–1914. [DOI] [PubMed] [Google Scholar]
- Tseng D., Rachakonda L., Su Z., Advani R., Horning S., Hoppe R., et al. (2012) Interim-treatment quantitative PET parameters predict progression and death among patients with Hodgkin’s disease. Radiat Oncol 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W. (2007) 18F-FDG PET in non-Hodgkin’s lymphoma: qualitative or quantitative? J Nucl Med 48: 1580–1582. [DOI] [PubMed] [Google Scholar]
- Yeh J., Diller L. (2012) Pediatric Hodgkin lymphoma: trade-offs between short- and long-term mortality risks. Blood 120: 2195–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes A., Connors J., Park S., Fanale M., O’Meara M., Hunder N., et al. (2013) Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol 14: 1348–1356. [DOI] [PubMed] [Google Scholar]
- Zinzani P., Bonfichi M., Rossi G., Zaja F., Vitolo U., Pavone V., et al. (2013) Early salvage with high-dose chemotherapy and stem cell transplantation in advanced stage Hodgkin’s lymphoma patients with positive positron emission tomography after two courses of chemotherapy: preliminary results of the iil-hd0801 study. Haematologica 98: 6. [Google Scholar]
- Zinzani P., Rigacci L., Stefoni V., Broccoli A., Puccini B., Castagnoli A., et al. (2012) Early interim 18F-FDG PET in Hodgkin’s lymphoma: evaluation on 304 patients. Eur J Nucl Med Mol Imaging 39: 4–12. [DOI] [PubMed] [Google Scholar]
- Zinzani P., Tani M., Fanti S., Alinari L., Musuraca G., Marchi E., et al. (2006) Early positron emission tomography (PET) restaging: a predictive final response in Hodgkin’s disease patients. Ann Oncol 17: 1296–1300. [DOI] [PubMed] [Google Scholar]