Abstract
Background
We aimed to analyze the association between hypertension and deep vein thrombosis (DVT) after orthopedic surgery.
Methods
Relevant studies were identified by a search of PubMed, Embase, China National Knowledge Infrastructure, Wanfang, the Chinese Biomedical Literature, and Weipu database until December 2015. The association between hypertension and DVT after orthopedic surgery was assessed by pooled odds ratios (ORs) and 95 % confidence intervals (CIs). Heterogeneity was evaluated by the Chi-square test based on Q statistic and I2 statistics. Finally, publication bias was evaluated by Egger’s test.
Results
A total of 16 articles with 68,955 males and 53,057 females were eventually identified. Studies yielded effects for homogeneous (Q = 38.41, P = 0.0008, and I2 = 60.9 %). Meta-analysis showed that hypertension was associated with DVT orthopedic surgery (OR 2.89, 95 % CI 2.18–3.83, Z = 7.38, P < 0.05). No statistical evidence of publication bias was found among studies (t = 1.90, P = 0.08). The funnel plot was symmetry, and the results were reliable.
Conclusions
Hypertension may promote DVT after orthopedic surgery, and may be an important risk factor of DVT occurrence.
Keywords: Meta-analysis, Hypertension, Deep vein thrombosis
Background
Hypertension is one of the major causes of disease burden all over the world [1]. In 2000, it was estimated that approximately 1 billion cases suffered hypertension and by 2025, the number is predicted to increase to 1.56 billion [2]. It is one of the most important risk factors for heart disease, stroke, coronary artery disease, and premature death [3]. Obesity, smoking, alcohol consumption, age, and education have been reported to play important roles in the risk of untreated and uncontrolled hypertension [4–6].
Deep vein thrombosis (DVT) is a systemic disease with a incidence of 67 per 100,000 of cases every year [7]. DVT could lead to postphlebitic syndrome, pulmonary embolism, and even death. In spite of adequate treatment, 1–8 % of patients developing pulmonary embolization will die [8, 9] and others will undergo long-term complications including chronic thromboembolic pulmonary hypertension and postphlebitic syndrome [10]. DVT is commonly associated with several co-morbidities. Over the past several years, studies on the association between DVT and hypertension have been reported, but the results are inconsistent. Some studies verified that hypertension could increase the development of DVT [11, 12]. However, Wang et al. [13] and Song et al. [14] reported that there was no statistically significant correlation between DVT and hypertension. Therefore, the controversial issue remains to be investigated.
Thus, in the current study, we performed a meta-analysis of available eligible studies to better elucidate the association between hypertension and DVT after orthopedic surgery.
Methods
The paper did not involve any human or animal study, so the ethical approval was not required.
Literature search
We searched electronic databases PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Embase (http://www.embase.com), China National Knowledge Infrastructure (CNKI, http://www.cnki.net/), Wanfang (http://g.wanfangdata.com.cn/), the Chinese BioMedical Literature (CBM, http://www.sinomed.ac.cn/), and Weipu database (http://www.cqvip.com/) updated to December 2015 for all the publications on the association between hypertension and DVT. The search terms were hypertension or high blood pressure or HBP; deep vein thrombosis or thrombose veineuse profonde or DVT or deep venous thrombosis; orthopedic post-operation or orthopedic or orthopaedic and postoperative. Language restrictions were not used for the search.
Study selection
Studies were included if they met the following criteria: (1) the observation group was patients with DVT after orthopedic surgery and the control group was patients without DVT after orthopedic surgery; (2) published on association between hypertension (blood pressure > 140/90 mm Hg) and DVT after orthopedic surgery in Chinese or English; (3) the number of patients with hypertension in the observation group and control group could be obtained. Studies were excluded if they were reviews, reports, or letters.
Data extraction
With the standard protocol, two investigators independently extracted the following data from the included studies: the first author, publication year, study time and region, the number of patients in control group or observation group, the number of patients with hypertension, and the demographic characteristics (sex, age, hyperlipidemia, and diabetes mellitus). Disagreements were resolved through discussion or settled by a third reviewer.
Statistical analysis
Meta-analysis was carried out using R 3.12 software. The odds ratio (OR) and its 95 % confidence interval (CI) were calculated for effect index. Heterogeneity test was evaluated by Chi-square based on Q statistic [15] and I2 statistics [16]. A random effects model was used to combine the data for the heterogeneous outcomes (P < 0.05 or I2 ≥ 50 %); otherwise, a fixed effects model was used [17]. A sensitivity analysis was performed, in which one study was removed at a time and others were analyzed to examine the influence of a single study on the combined OR value [18]. Publication bias was evaluated through funnel plot visual analysis with the Egger’s tests [19, 20]. A P value less than 0.05 was considered statistically significant.
Results
Characteristics of included studies
The process of study selection was shown in Fig. 1. Initially, a total of 124 potentially relevant articles were retrieved from the databases (PubMed 18; Embase 11; CNKI 8; Wanfang 80; Weipu 2; CBM 5). Then, 108 articles were left after eliminating the duplicate publication, and 77 of them were excluded after screening the title and abstract. As a consequence, 31 articles were left and 15 (3 review, 1 letter, 3 case-report, 2 repeated people, and 6 did not provide sufficient data) of them were excluded after screening the full text. Finally, 16 articles [11, 12, 21–34] including 68,955 males and 53,057 females were included in this meta-analysis (Table 1). These studies were published between 2009 and 2015 with researches done between 2005 and 2014.
Fig. 1.
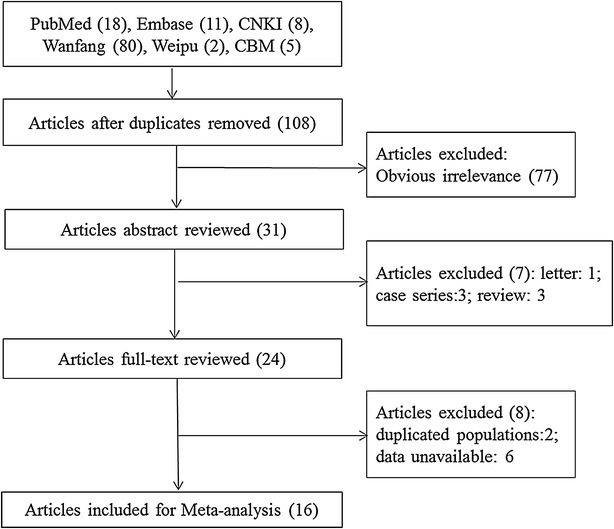
Flow diagram of study selection
Table 1.
Characteristics of included studies in the meta-analysis
| Author | Public year | Study year | Study location | DVT | No. | Sex (M/F) | Age | Hyperlipidemia | Diabetes mellitus | Hypertension |
|---|---|---|---|---|---|---|---|---|---|---|
| Ma Jun | 2009 | 2007.2–2007.7 | Sichuan | Yes | 17 | 4/13 | 15 (≥65) | 15a | 4 | 6 |
| No | 34 | 9/25 | 16 (≥65) | 7a | 5 | 5 | ||||
| Zhang Ke-yun | 2014 | 2010.2–2012.1 | Hunan | Yes | 28 | 6/22 | 28 (≥65) | 24a | 6 | 8 |
| No | 64 | 17/47 | 30(≥65) | 17a | 10 | 12 | ||||
| Wu Fang-li | 2011 | 2008.5–2009.12 | Zhejiang | Yes | 15 | 6/9 | 70.08 ± 12.18 | NA | NA | 9 |
| No | 171 | 36/135 | 63.83 ± 10.6 | NA | NA | 36 | ||||
| Zheng Gui-juan | 2015 | 2013.1–2014.6 | Beijing | Yes | 16 | 3/13 | 14 (≥65) | 14a | 6 | 5 |
| No | 36 | 12/24 | 16 (≥65) | 9a | 4 | 7 | ||||
| He Han-liang | 2014 | 2011.1–2013.12 | Zhejiang | Yes | 203 | 134/69 | 57 (>60) | 73 | NA | 115 |
| No | 3967 | 2399/1771 | 833 (>60) | 1019 | NA | 1940 | ||||
| Yao Jie | 2013 | NA | Ningxia | Yes | 212 | 145/67 | 76 (>64) | 113a | 20 | 43 |
| No | 4921 | 3012/1909 | 854 (>64) | 1113a | 152 | 286 | ||||
| Rong Jin-yang | 2013 | 2009.1–2013.4 | Shanxi | Yes | 130 | 76/54 | 48 (28–70) | 30 | 33 | 48 |
| No | 134 | 78/56 | 50 (30–72) | 9 | 10 | 13 | ||||
| Long Jiang | 2013 | 2009.5–2012.5 | Yunnan | Yes | 73 | NA | 49.3 ± 26.1 | 47 | 53 | 49 |
| No | 72 | NA | 23 | 37 | 25 | |||||
| Ya Jun | 2014 | 2010.3–2013.3 | Yunnan | Yes | 25 | 34/16 | 62.5 ± 1.2 | NA | 8 | 9 |
| No | 25 | NA | 6 | 4 | ||||||
| Sun Yong-fei | 2011 | 2005.5–2010.10 | Zhejiang | Yes | 70 | 30/40 | 45.5 ± 7.1 | NA | 18 | 20 |
| No | 70 | 30/40 | 45.6 ± 7.2 | NA | 8 | 9 | ||||
| Wang Da-wei | 2012 | 2007.4–2011.4 | Liaoning | Yes | 91 | 101/64 | 38.2 ± 8.23 | 29 | 19 | 23 |
| No | 74 | 6 | 6 | 8 | ||||||
| Wang Xiao-feng | 2013 | 2011.10–2012.11 | Guangdong | Yes | 52 | 64/39 | 59.8 ± 4.3 | 36 | 34 | 31 |
| No | 51 | 39.4 ± 3.9 | 15 | 19 | 14 | |||||
| Huang Kun | 2014 | 2010–2013 | Jiangsu | Yes | 80 | 57/23 | 51.3 ± 11.4 | 26 | 23 | 26 |
| No | 80 | 55/25 | 50.8 ± 10.2 | 9 | 14 | 12 | ||||
| Guo Chang-jun | 2013 | 2011.1–2013.1 | Zhejiang | Yes | 98 | 113/67 | 37.5 ± 1.2 | 22 | 28 | 22 |
| No | 82 | 9 | 10 | 8 | ||||||
| Yang Si-dong | 2015 | 2013.7–2014.7 | Hebei | Yes | 147 | 410/451 | 54 (15–87) | NA | 18 | 54 |
| No | 714 | NA | 66 | 161 | ||||||
| Zheng Sui-zhu | 2013 | 2011.10–2012.10 | Zhejiang | Yes | 58 | 30/28 | 68.7 ± 17.1 | NA | 36 | 25 |
| No | 62 | 32/30 | 55.6 ± 11.2 | NA | 8 | 10 |
aTriglyceride ≥ 1.7 mmol/L, M male, F female, DVT deep vein thrombosis, NA not available
Merging quantitative data
The homogeneity analysis exhibited good with heterogeneity test (Q = 38.41, P = 0.0008, and I2 = 60.9 %). Then, the random effects model was used for further analysis. Meta-analysis showed that hypertension was associated with DVT after orthopedic surgery (OR 2.89, 95 % CI 2.18–3.83, Z 7.38, P < 0.05, Fig. 2). Sensitivity analysis showed that our results were stable (OR 2.89, 95 % CI: 2.18-3.83, Fig. 3). After Egger’s regression test, no publication bias among studies was found (t = 1.90, P = 0.08). The funnel plot was symmetry, so there was no publication bias and the result was reliable (Fig. 4).
Fig. 2.
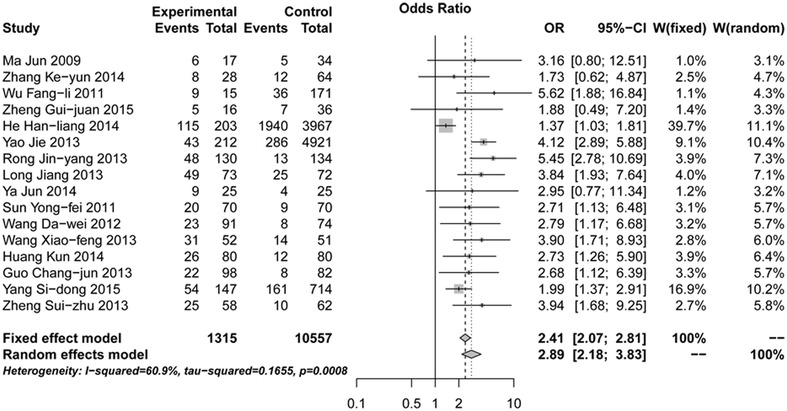
Forest plot of association between hypertension and deep vein thrombosis (DVT) after orthopedic surgery
Fig. 3.
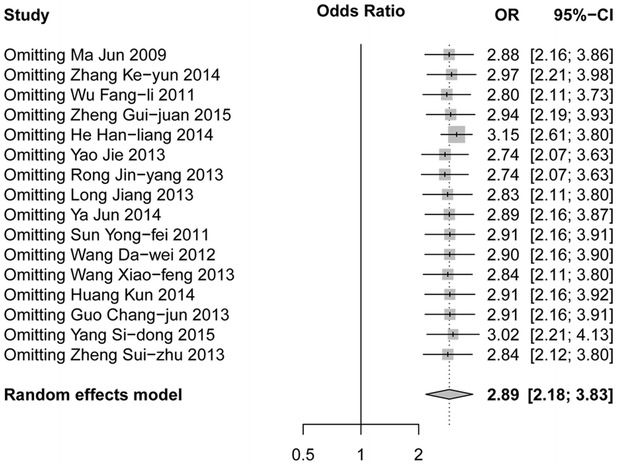
Forest plot for sensitivity analysis of association between hypertension and DVT after orthopedic surgery
Fig. 4.
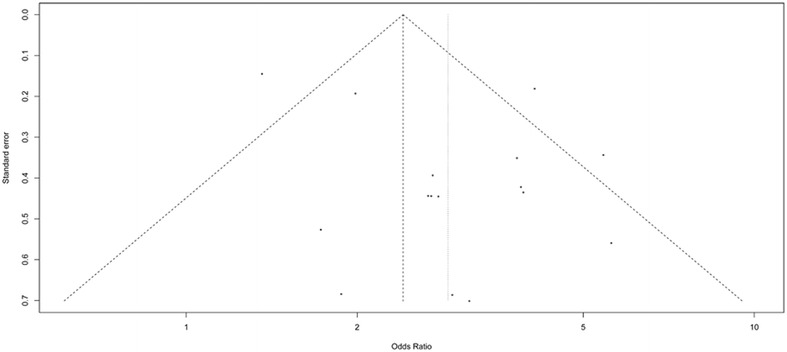
Funnel plot of association between hypertension and DVT after orthopedic surgery
Subgroup analysis
Subgroup analysis pointed out that heterogeneity was decreased to different degrees (Table 2). In addition, the results of meta-analysis in each subgroup showed that hypertension may promote the formation of DVT after orthopedic surgery.
Table 2.
Subgroup analyses of associations between hypertension and deep vein thrombosis after orthopedics surgery
| Classification | Item | N | I 2 (%) | P | Model | OR [95 % CI] |
|---|---|---|---|---|---|---|
| Regional distribution | Western China | 4 | 0 | 0.9498 | F | 3.89 [2.85–5.30] |
| Central China | 3 | 71.5 | 0.0298 | R | 2.70 [1.32–5.52] | |
| Eastern China | 9 | 53.9 | 0.0268 | R | 3.95 [2.92–5.33] | |
| The proportion of including | N < 500 | 13 | 0 | 0.8881 | F | 3.37 [2.63–4.31] |
| N ≥ 500 | 3 | 91.5 | <0.0001 | R | 2.23 [1.14–4.34] |
OR odds ratio, R random effects model, F fixed effect model
Discussion
This is the first systematic review and meta-analysis of studies, to our knowledge, examining the correlation between hypertension and DVT after orthopedic surgery. Totally 16 articles with 68,955 males and 53,057 females were included in this meta-analysis. The results showed that hypertension might promote DVT after orthopedic surgery (OR 2.89, 95 % CI 2.18–3.83, Z = 7.38).
In spite of the inherent risk of developing DVT for patients with orthopedic surgery, researches on risks of developing DVT were limited. Red blood cell storage has been found to be associated with increased incidence of DVT [35]. A previous report has indicated that patients with increased concentrations of factor VIII and von Willebrand’s factor have increased risk of DVT [36]. Compared with healthy controls, levels of red cell distribution width were higher in pre-hypertensive and hypertensive patients independently of age, inflammatory status, and anemia, suggesting the correlations between red cell distribution width and hypertension [37]. All of these may hint a potential relationship between DVT and hypertension.
The only risk factor of DVT in accordance with the conclusion from this meta-analysis is hypertension, which has already been verified previously. Several prospective studies have addressed the associations between hypertension and DVT. Patients with hypertension have been found with 2-fold increased likelihood of developing DVT [38]. In the current study, the results of meta-analysis in each subgroup have showed that hypertension may promote the formation of DVT after orthopedic surgery. In addition, hypertension has been found as an independent predictor of venous thromboembolism (VTE) in the general population [39]. In another study, after a prospective registry of 5451 patients with DVT, Goldhaber et al. [40] have found that 50 % patients have co-morbidities with hypertension. Kaisorn et al. [41] have also reported that hypertension may independently increase the risk of developing operative DVT (OR 1.785; 95 % CI 1.180–2.699; P = 0.006).
Age is a high prevalence of asymptomatic DVT event which has been identified in patients over 80 years [42]. A previous study with 102 consecutive patients of follow-up found that age greater than 65 years, body mass index (BMI) > 30 kg/m2, and smoking were risk factors for DVT [43]. In a recent study comprising 87 574 individuals found that obesity was a causal risk factor for DVT [44]. Examining on VTE, Chamberlain et al. [45] found that low-density lipoprotein cholesterol was not an risk factor of VTE. Another study with 855 men (65 VTE events) identified that smoking and waist circumference were risk factors for VTE, whereas high cholesterol and hypertension were not [46]. In addition, a Copenhagen City Heart Study pointed out that hypertension, smoking, and obesity were important risk factors for VTE, whereas total/high-density lipoprotein/low-density lipoprotein cholesterol, triglyceride, and diabetes mellitus were not [47].
Some limitations of this study should be addressed. First, only published studies were included and publication bias might exist, although no significant bias was detected by Egger’s test. Second, significant heterogeneity across studies was presented in overall and subgroup analysis, which might influence the pooled results. Third, although there was no limitation for language, only Chinese population was included, which might lead to bias. Fourth, as limited researches included in this meta-analysis, association between DVT and age, BMI, or gender was not analyzed. Finally, the small sample size was still insufficient to obtain a conclusive result. However, larger and well-designed studies based on different populations are warranted to validate our results.
In conclusion, this study showed that hypertension might promote DVT after orthopedic surgery, and it might be an important risk factor of DVT occurrence.
Authors’ contributions
LH participated in the design of this study and performed the statistical analysis. JL carried out the study and collected important background information. YJ drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Lei Huang, Email: hlbone@163.com.
Jie Li, Email: Lijie7107@163.com.
Yong Jiang, Phone: +86-0411-83635963-7108, Email: jiangyongshh@hotmail.com.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)70151-3. [DOI] [PubMed] [Google Scholar]
- 3.Organization WH. World health statistics 2012 [internet] Genebra: WHO; 2012. [Google Scholar]
- 4.Danon-Hersch N, Marques-Vidal P, Bovet P, Chiolero A, Paccaud F, Pecoud A, Hayoz D, Mooser V, Waeber G, Vollenweider P. Prevalence, awareness, treatment and control of high blood pressure in a Swiss city general population: the CoLaus study. Eur J CardioL Prev Rehabil. 2009;16(1):66–72. doi: 10.1097/HJR.0b013e32831e9511. [DOI] [PubMed] [Google Scholar]
- 5.Tian S, Dong G-H, Wang D, Liu M-M, Lin Q, Meng X-J, Xu L-X, Hou H, Ren Y-F. Factors associated with prevalence, awareness, treatment and control of hypertension in urban adults from 33 communities in China: the CHPSNE study. Hypertens Res. 2011;34(10):1087–1092. doi: 10.1038/hr.2011.99. [DOI] [PubMed] [Google Scholar]
- 6.Agyemang C, Van Valkengoed I, Koopmans R, Stronks K. Factors associated with hypertension awareness, treatment and control among ethnic groups in Amsterdam, the Netherlands: the SUNSET study. J Hum Hypertens. 2006;20(11):874–881. doi: 10.1038/sj.jhh.1002073. [DOI] [PubMed] [Google Scholar]
- 7.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23):I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 8.Prandoni P, Lensing AW, Prins MR. Long-term outcomes after deep venous thrombosis of the lower extremities. Vasc Med. 1998;3(1):57–60. doi: 10.1177/1358836X9800300112. [DOI] [PubMed] [Google Scholar]
- 9.Hirsh J, Bates SM. Prognosis in acute pulmonary embolism. Lancet. 1999;353(9162):1375–1376. doi: 10.1016/S0140-6736(99)90053-3. [DOI] [PubMed] [Google Scholar]
- 10.Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med. 2004;164(1):17–26. doi: 10.1001/archinte.164.1.17. [DOI] [PubMed] [Google Scholar]
- 11.Chang-jun G. Risk factors of deep venous thrombosis after traumatic fracture. J N Pharm. 2013;8:99–100. [Google Scholar]
- 12.Han-liang H. Risk factors of deep venous thrombosis after orthopaedic surgery. Chin J Surg Integr Tradit West Med. 2014;6:593–595. [Google Scholar]
- 13.Wang C-J, Wang J-W, Chen L-M, Chen H-S, Yang B-Y, Cheng S-M. Deep vein thrombosis after total knee arthroplasty. J Formos Med Assoc. 2000;99(11):848–853. [PubMed] [Google Scholar]
- 14.Song EK, Kim JK, Lee KB, Seon JK. Deep vein thrombosis after total knee replacement: incidence and correlation with clinical risk factors. J Korean Knee Soc. 1998;10(1):18–22. [Google Scholar]
- 15.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127(9):820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327(7414):557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng R-N, Zhao C, Sun C-H, Li Y. Meta-analysis of TNF 308 G/A polymorphism and type 2 diabetes mellitus. PLoS One. 2011;6(4):e18480. doi: 10.1371/journal.pone.0018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu ZH, Ding YL, Xiu LC, Pan HY, Liang Y, Zhong SQ, Liu WW, Rao SQ, Kong DL. A meta-analysis of the association between TNF-alpha -308G > A polymorphism and type 2 diabetes mellitus in Han Chinese population. PLoS One. 2013;8(3):e59421. doi: 10.1371/journal.pone.0059421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Da-wei W. Risk factors analysis of deep vein thrombosis in orthopaedic trauma patients. Seek Med Ask Med. 2012;10(4):10. [Google Scholar]
- 22.Fang-li W, Yan-feng M. Assessment of risk factors for deep vein thrombosis after total joint arthroplasty. J Med Res. 2011;40(5):109–111. [Google Scholar]
- 23.Gui-juan Z, Jia G. The influence factors of lower extremities deep venous thrombosis after hip replacement. Chin J Prim Med Pharm. 2015;22(13):1959–1963. [Google Scholar]
- 24.Jiang L. Occurrence and risk factors of deep venous thrombosis after traumatic fracture. Seek Med Ask Med. 2013;11:03. [Google Scholar]
- 25.Jie Y, Jin-hai M, Wen-juan W, et al. Risk factors for deep vein thrombosis in lower extremity after orthopedic surgery. Chin J Anesthesiol. 2013;33(4):413–416. [Google Scholar]
- 26.Jin-yang R. Risk factors analysis of deep vein thrombosis after orthopaedic trauma. Med Forum. 2013;29:3873–3874. [Google Scholar]
- 27.Jun M, Bin S, Jing Y, et al. Risk factors for deep vein thrombosis after total hip arthroplasty. Orthop J Chin. 2009;17(13):965–969. [Google Scholar]
- 28.Jun Y, Zhi-jian M, Peng L, et al. Factors analysis and clinical treatment of the patients after surgery with deep venous thrombosis. Chin Med Herald. 2014;11(10):25–27. [Google Scholar]
- 29.Ke-yun Z, Li-ming Y, Xu-hua Z, et al. Analyze the relationship between hip replacement and deep vein thrombosis. Guide Chin Med. 2014;14:34–35. [Google Scholar]
- 30.Kun H. Risk factors of deep venous thrombosis after orthopaedic trauma of 80 cases. All For Health. 2014;8(6):128–129. [Google Scholar]
- 31.Xiao-feng W. Risk factors of deep venous thrombosis after orthopaedic trauma of 103 cases. Guide Chin Med. 2013;30:429–430. [Google Scholar]
- 32.Yang SD, Liu H, Sun YP, Yang DL, Shen Y, Feng SQ, Zhao FD, Ding WY. Prevalence and risk factors of deep vein thrombosis in patients after spine surgery: a retrospective case-cohort study. Sci Rep. 2015;5:11834. doi: 10.1038/srep11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yong-fei S, Zhi-hong L. Research analysis of risk factors analysis of deep vein thrombosis in orthopaedic trauma patients. Chin Modern Doct. 2011;49(18):255–256. [Google Scholar]
- 34.Sui-zhu Z, Yiing-ying W, Qian-qian J. Related risk factors analysis and nursing strategy of deep venous thrombosis after orthopaedic surgery. Chin J Gen Prac. 2013;11(11):1806–1807. [Google Scholar]
- 35.Spinella PC, Carroll CL, Staff I, Gross R, Quay JM, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13(5):R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tirado I, Mateo J, Soria JM, Oliver A, Martínez-Sánchez E, Vallvé C, Borrell M, Urrutia T, Fontcuberta J. The ABO blood group genotype and factor VIII levels as independent risk factors for venous thromboembolism. Thromb Haemost. 2005;93(3):468–474. doi: 10.1160/TH04-04-0251. [DOI] [PubMed] [Google Scholar]
- 37.Tanindi A, Topal FE, Topal F, Celik B. Red cell distribution width in patients with prehypertension and hypertension. Blood Press. 2012;21(3):177–181. doi: 10.3109/08037051.2012.645335. [DOI] [PubMed] [Google Scholar]
- 38.Semrad TJ, O’Donnell R, Wun T, Chew H, Harvey D, Zhou H, White RH. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg. 2007;106(4):601–608. doi: 10.3171/jns.2007.106.4.601. [DOI] [PubMed] [Google Scholar]
- 39.Goldhaber SZ. Risk factors for venous thromboembolism. Am J Cardiol. 2010;56(1):1–7. doi: 10.1016/j.jacc.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 40.Goldhaber SZ, Tapson VF, Committee DFS. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol. 2004;93(2):259–262. doi: 10.1016/j.amjcard.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 41.Chaichana KL, Pendleton C, Jackson C, Martinez-Gutierrez JC, Diaz-Stransky A, Aguayo J, Olivi A, Weingart J, Gallia G, Lim M. Deep venous thrombosis and pulmonary embolisms in adult patients undergoing craniotomy for brain tumors. Neurol Res. 2013;35(2):206–211. doi: 10.1179/1743132812Y.0000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oger E, Bressollette L, Nonent M, Lacut K, Guias B, Couturaud F, Leroyer C, Mottier D. High prevalence of asymptomatic deep vein thrombosis on admission in a medical unit among elderly patients. Thromb Haemost. 2002;88(4):592–597. [PubMed] [Google Scholar]
- 43.Delis K, Hunt N, Strachan R, Nicolaides A. Incidence, natural history and risk factors of deep vein thrombosis in elective knee arthroscopy. Thromb Haemost. 2001;86(3):817–821. [PubMed] [Google Scholar]
- 44.Klovaite J, Benn M, Nordestgaard B. Obesity as a causal risk factor for deep venous thrombosis: a Mendelian randomization study. J Intern Med. 2015;277(5):573–584. doi: 10.1111/joim.12299. [DOI] [PubMed] [Google Scholar]
- 45.Chamberlain AM, Folsom AR, Heckbert SR, Rosamond WD, Cushman M. High-density lipoprotein cholesterol and venous thromboembolism in the longitudinal Investigation of thromboembolism etiology (LITE) Blood. 2008;112(7):2675–2680. doi: 10.1182/blood-2008-05-157412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansson P-O, Eriksson H, Welin L, Svärdsudd K, Wilhelmsen L. Smoking and abdominal obesity: risk factors for venous thromboembolism among middle-aged men: the study of men born in 1913. Arch Intern Med. 1999;159(16):1886–1890. doi: 10.1001/archinte.159.16.1886. [DOI] [PubMed] [Google Scholar]
- 47.Holst AG, Jensen G, Prescott E. Risk factors for venous thromboembolism results from the Copenhagen City Heart Study. Circulation. 2010;121(17):1896–1903. doi: 10.1161/CIRCULATIONAHA.109.921460. [DOI] [PubMed] [Google Scholar]


