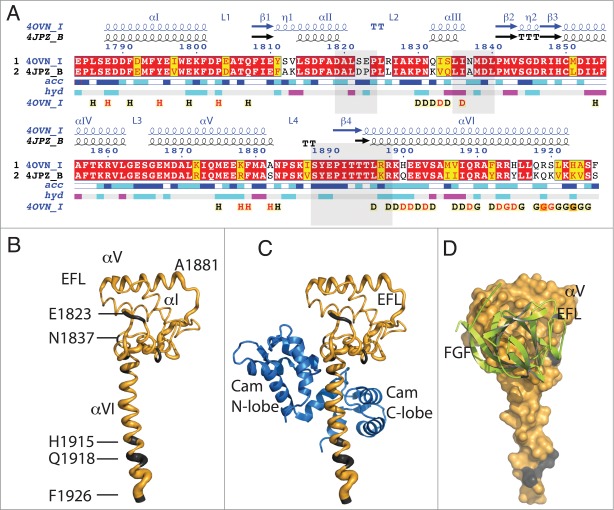
Figure 1.
Sequence and structural similarity of Nav1.5 and Nav1.2 in their cytoplasmic C-terminal region. (A) Red background displays sequence identity, yellow background sequence conservation of charge and white background, non-homologous residues. The relative accessibility (acc), calculated by Endspript/DSSP, is shown in white for buried residues and in blue for accessible residues. 39 Residues involved in the intermolecular contact between 2 Nav1.5 molecules are labeled “H” (contacts with distances less 3.2 Å, black H; those with distances between 3.2 and 5.0 Å, red H). Helix αIII interacts with the CaM N-lobe and helix αVI with the CaM C-lobe (“D”; colored as for the “H”). Residues at the end of helix αVI involved in intermolecular contact between two Nav1.5 molecules (in CTNav1.5-CaM) or with CaM N-lobe in CTNav1.5-CaMCa-FHF are labeled “G.” The end of helix αVI interacts with another molecule of Nav1.5. The light gray boxes show the residues of Nav1.2 that interact with FHF in the ternary complex (PDB ID 4JPZ). (B) Cα trace of CTNav1.5 in which the thickness of the tube is proportional to the rmsd of the Cα residues in the structural overlap of CTNav1.5 (PDB ID 4OVN) with the CTNav1.2 (PDB ID 4JPZ); (non-conserved residues in gray). (C) As (B) with CaM bound as in 4OVN. (D) Surface representation of the Nav1.5 showing complete sequence conservation at the surface that binds FHF (green); Nav1.5 is 180 degrees from the orientation in (B and C).