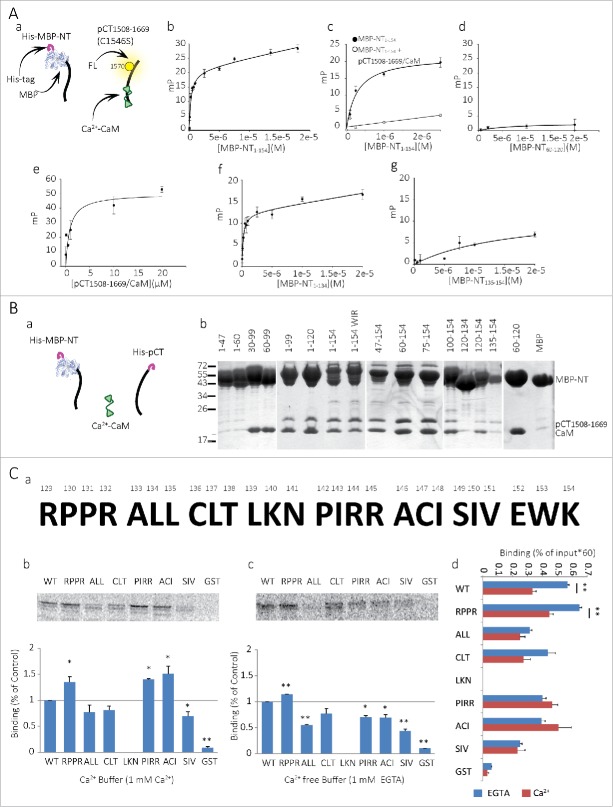
Figure 4 (See previous page).
The NT-pCT interaction site in the NT. (A) Binding isotherms of CaV1.2 MBP-NT proteins with a fluorescein-labeled pCT1508-1669/CaM complex measured by fluorescence polarization (FP). a. Schematic illustration of the fluorescein labeled ‘bait’ and MBP fused ‘target’ molecules used in the assay (label is on pCT1508-1669 C1570). b. Full length MBP-NT1-154 binds pCT1508-1669 with KD = 0.29 ± 0.05 μM. c. MBP-NT1-154 binding is specific to pCT1508-1669/CaM. FP signal is abolished with the addition of excess unlabeled (‘cold’) pCT1508-1669/CaM (5 μM, 100 fold higher than labeled protein). d. No binding was observed for MBP-NT60-120. e. Binding is also observed for a converse FP experiment where a fluorescein labeled NT47-154(C136S) is titrated with the pCT1508-1669/CaM complex. f. MBP-NT1-134 binds pCT1508-1669/CaM with KD = 0.21 ± 0.06 μM. g. MBP-NT135-154 only weakly binds pCT1508-1669/CaM with KD =16.67 ± 28.58 μM. All measurements were performed at 10° C. Solutions contained 1 mM CaCl2 and 25% glycerol. Fitting model assumed a single binding site + non-specific binding. mP denotes the change from baseline (ΔP) polarization in thousandth FP unit. (B) Pull-down experiment of pCT1508-1669 and CaM by MBP-fused NT fragments. a. Schematic illustration of the proteins used: His-MBP-NTs, CaM and His-pCT. b. Final elution from amylose resin used for immobilization is presented. (C) a. NT 129-154 mutations arranged in triples or quadruples which were mutated to alanines. b-c. Top, pull down experiments of radioactive labeled NTs, pulled by GST-pCT in 1 mM Ca2+ (b) or 1 mM EGTA (c). Bottom, average of 3 of pull down experiments normalized to WT NT of each experiment. * P < 0.05, ** P < 0.01. d. Non-normalized average of 3 pull down experiments with EGTA (blue bars) or 1 mM Ca2+ (dark red bars). ** P < 0.01.