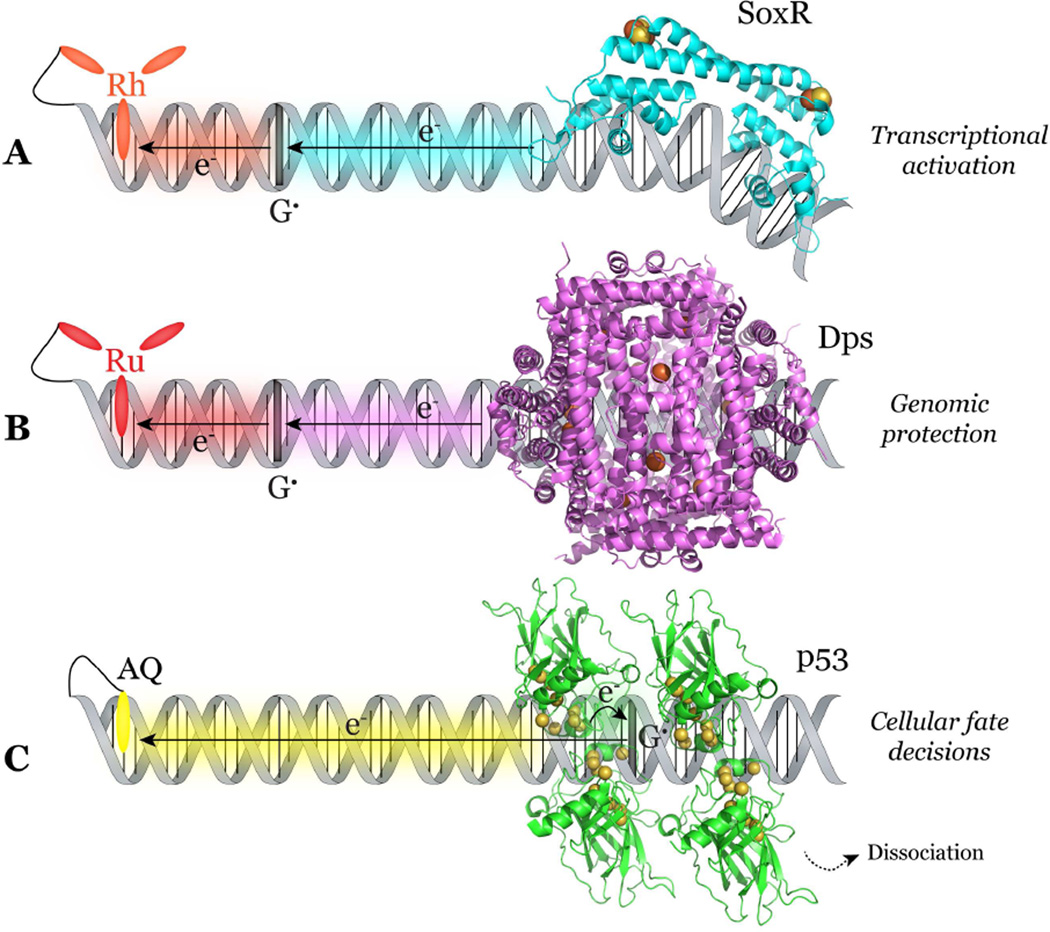
Figure 8. SoxR, Dps and p53 proteins use DNA CT to sense and respond to distant DNA damage produced by a tethered photooxidant.
(A) SoxR, a bacterial transcription factor involved in response to superoxide stress, contains [2Fe2S]2+/1+ clusters and undergoes a conformational change and transcriptional activation upon oxidation. Irradiation of a construct with covalently tethered photooxidant [Rh(phi)2(bpy′)]3+ located 80 base pairs from the SoxR promoter binding site produced soxS, the target gene for SoxR. Irradiation of the photooxidant produces an excited state capable of oxidizing DNA, the injected electron hole localizes to guanine radicals, and SoxR then becomes oxidized to fill guanine radicals, resulting in transcriptional activation from a distance via DNA CT. (B) Dps proteins, bacterial mini ferritins implicated in the virulence of pathogenic bacteria, contain iron-binding ferroxidase sites and are involved in DNA protection from oxidative stress. Damage created at a low redox potential guanine triplet upon irradiation with tethered photooxidant [Ru(phen)(dppz)(bpy′)]2+ can be attenuated by Dps loaded with ferrous iron, but not by Apo-Dps or Dps loaded with ferric iron, which both lack reducing equivalents. Charge transfer from the ferrous iron bound at the ferroxidase sites of Dps to guanine radical holes within DNA could be an efficient mechanism of genomic protection from a distance via DNA CT. (C) p53, a human transcription factor known as the “guardian of the genome”, contains a network of redox-active cysteine residues. Irradiation of constructs with a tethered anthraquinone photooxidant separated from the p53 response element can result in oxidation of these cysteine residues and dissociation of p53 from the DNA. Specifically, when the response element contains low redox potential guanine sites, p53 can become efficiently oxidized via DNA CT, resulting in decisions regarding the cellular fate. PDB files: SoxR (2ZHG), Dps (1N1Q), p53 (3KMD).