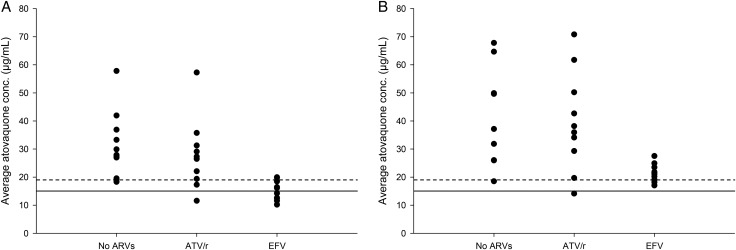
Figure 2.
Average atovaquone plasma concentrations (Cavg) in human immunodeficiency virus-infected patients receiving no antiretroviral therapy (no ARVs), atazanavir/ritonavir (ATV/r), or efavirenz (EFV) in combination with atovaquone 750 mg twice daily for 14 days (A) or 1500 mg twice daily for 14 days (B). Horizontal lines: solid line represents the Cavg previously associated with successful treatment of Pneumocystis jiroveci pneumonia (15 µg/mL); the dashed line represents the Cavg previously associated with successful treatment of toxoplasma encephalitis (18.5 µg/mL).