Figure 1.
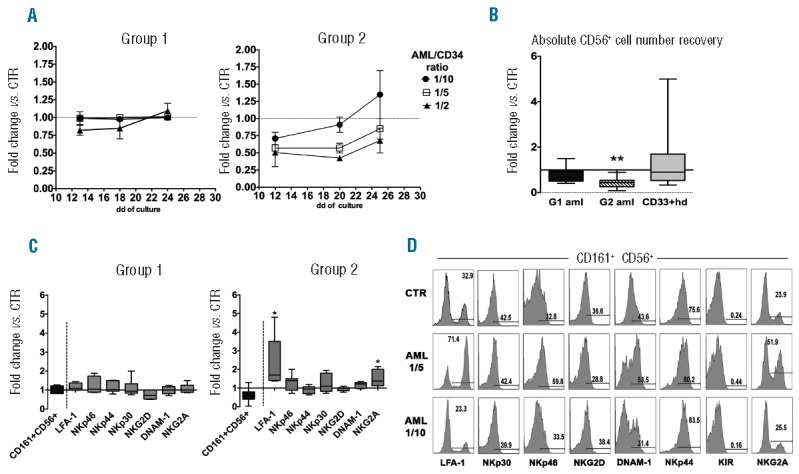
Modulation of in vitro NK-cell differentiation from CD34+ precursors by AML blasts. UCB-derived CD34+ cells were cultured in trans-well plates with medium containing Stem Cell Factor, FMS-like tyrosine kinase 3 ligand, IL-7, IL-15 and IL-21 (cytokine mix medium), in the absence (CTR) or in the presence of human AML blasts. (A) At different time intervals, cells were analyzed for CD161 and CD56 surface expression. Graphics represent the fold change (mean±SEM) of percentages of CD161+CD56+ cells recovered in the presence of AML blasts in comparison with controls, arbitrarily normalized to one. Group 1: 4 independent experiments performed with three different AML. Group 2: 3 independent experiments performed with two different AML. (B) Box and Whisker plot represents the CD161+CD56+ absolute cell number fold changes in cultures performed in the presence of Group 1 AML (8 independent experiments performed with five different G1 AML), Group 2 AML (8 independent experiments performed with four different G2 AML), or in the presence of HD-CD33+ myeloid cells (15 independent experiments performed with 15 different donors) compared to CTR, arbitrarily normalized to one. All cells were plated at 1/5 AML/CD34+ cell ratio. Wilcoxon signed rank test (**P≤0.005). (C) Box and Whisker plot represents the percentages fold change of CD161+CD56+ cells (black box) or CD161+CD56+ NK receptor-positive cells (gray box) after 25 days of co-culture performed in the presence of Group 1 or Group 2 AML (1/5 ratio) in comparison with CTR (normalized to one). Wilcoxon signed rank test (*P≤0.05). (D) Flow-cytometry assays were performed after 25 days of culture to analyze the expression of NK-specific receptors on CD161+CD56+ cells derived from CTR cultures, or from co-cultures with G2 AML at 1/5 and 1/10 AML/CD34+ cell ratio.
