Figure 3.
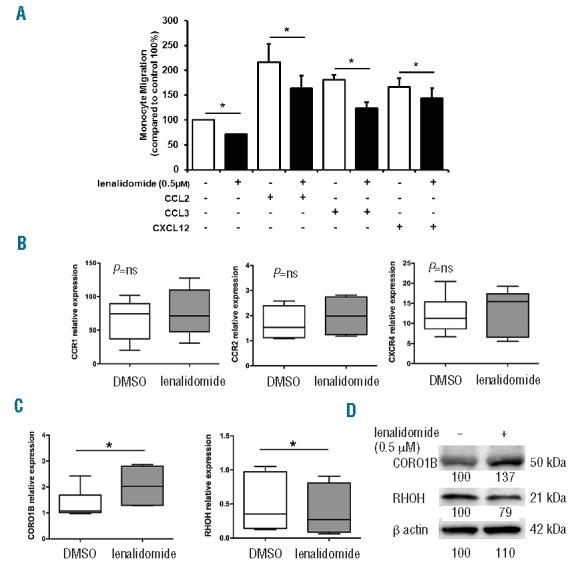
Lenalidomide reduces migration of monocytes from CLL patients. (A) Monocytes purified from 5 CLL patients were loaded in the upper chamber of 5-mm pore PET inserts with the addition of 0.5 μM lenalidomide or vehicle (DMSO). CCL2 (10 ng/mL), CCL3 (10 ng/mL), or CXCL12 (200 ng/mL) were added in the bottom chamber as chemoattractants. Monocytes were allowed to migrate for 4 h and migrated cells were quantified by fluorescence plate reader. Data are normalized on control (100%, DMSO-treated monocytes). Histograms represent mean±SEM of 5 independent experiments (Student t-test, *P<0.05). (B and C) Monocytes purified from 6 CLL patients were cultured in 24-well plates with 0.5 μM lenalidomide or vehicle (DMSO) for 4 h. (B) Gene expression of CCR1, CCR2, CXCR4, and (C) gene expression of RhoH and CORO1B, were measured by quantitative reverse-transcription PCR. Results of 2 independent experiments with 6 patient samples are presented as box plots; whiskers show min and max values. Lenalidomide does not modify CCR1, CCR2 and CXCR4 expression on CLL monocytes. Conversely, CORO1B is significantly up-regulated and RhoH down-regulated by lenalidomide treatment (Student t-test, *P<0.05). (D) Immunoblots show CORO1B and RHOH quantities after treatment with lenalidomide 0.5 μM in a representative sample of CLL monocytes. Densitometric quantification of bands normalized to the untreated control is shown below the immunoblots.
