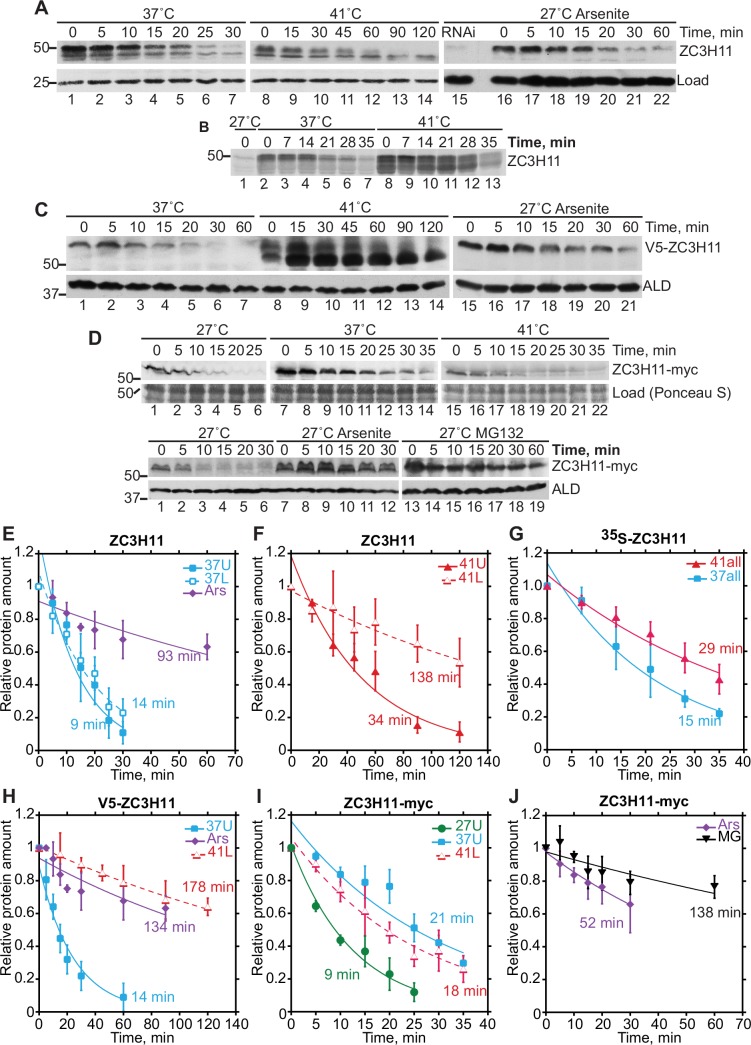
Fig 2. ZC3H11 protein becomes more stable upon heat shock.
A. Half-life of ZC3H11 protein. Procyclic cells were either heat-shocked at 37°C (lanes 1–7) or 41°C (lanes 8–14), or incubated with 10μM sodium arsenite for 1 hour (lanes 15–22), then treated with cycloheximide (100μg/ml) to shutoff translation. Samples were analyzed by Western blotting. One representative blot is shown; a stable cross-reacting band migrating elsewhere on the gel is the loading control. The signals from the two bands were quantified independently by densitometry. Cells with RNAi (lane 15) serve as a control to show the lack of any cross-reacting proteins co-migrating with the "lower" ZC3H11 band. B. Half-life of ZC3H11 protein measured by [35S]-methionine pulse-chase. Procyclic cells were heat-shocked at 37°C or 41°C for 1h, incubated in methionine-free labelling medium for 15min, [35S]-methionine was added for 20min, then the cells were centrifuged and resuspended in full medium at the same temperatures. ZC3H11 was immunoprecipitated and the labelled protein detected by autoradiography. Quantitation was by scanning the autoradiogram. C. Half-life of in situ-tagged V5-ZC3H11 protein. Procyclic cells expressing V5-ZC3H11 from a modified endogenous locus were incubated either at 37°C or 41°C for 1 hour and processed as for (A). Detection was with anti-V5 antibodies and to aldolase as loading control. The V5 tag adds about 1.5 kDa to the protein molecular weight but seems to have a greater effect on migration of ZC3H11 in polyacrylamide gels. D. Half-life of ZC3H11-myc protein. Procyclic cells expressing ZC3H11-myc from a modified endogenous locus (native 3'-UTR was changed to actin 3’-UTR as a result of C-terminal in situ tagging) were grown at 27°C, and incubated either at 37°C or 41°C, or with 10μM sodium arsenite or 10μg/ml MG132 (MG) for 1 hour and processed as for (A). Detection was with anti-myc antibodies and Ponceau S staining is shown as loading control. The myc tag adds about 3 kDa to the protein molecular weight. E, F. Results from (A). Results are the mean ± standard deviation for three biological replicates were plotted and exponential curves were fitted to the mean values in Kaleidograph. The upper ZC3H11 band is designated "U" and the lower one "L". Ars = arsenite. G. Results from (B); for details see E,F. H. Results from (C); for details see E,F. I, J. Results from (D); for details see E,F. MG = MG132.