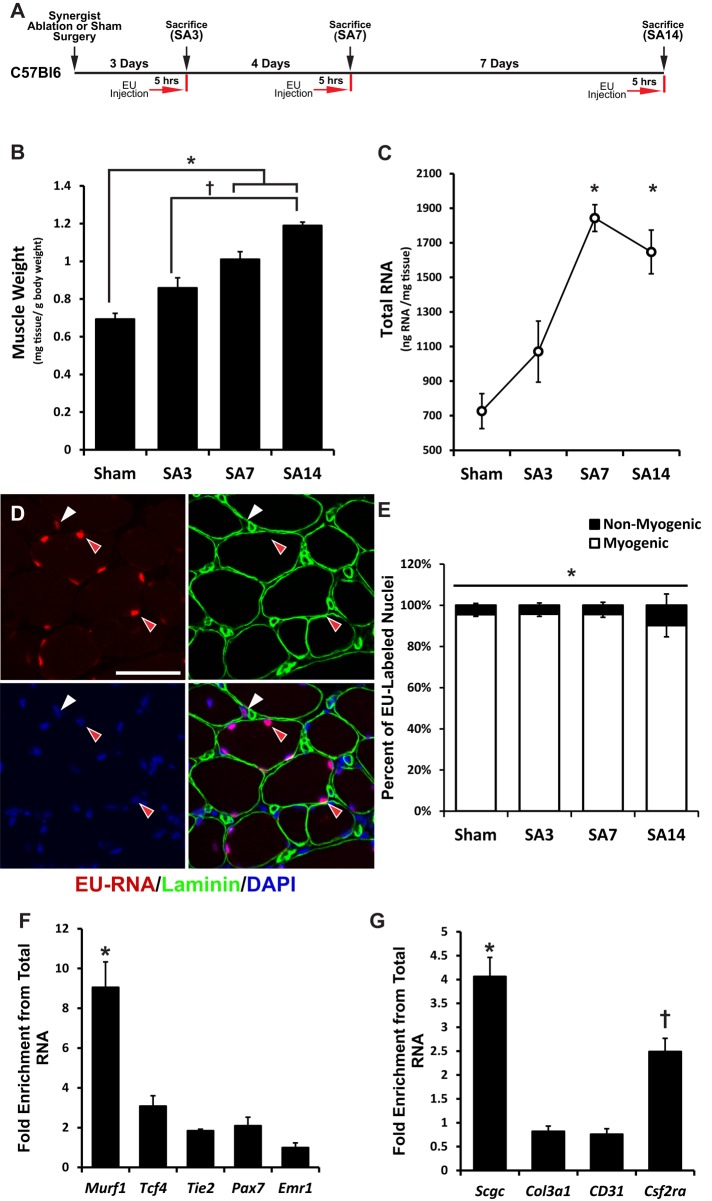
FIGURE 1:
Mechanical overload results in a progressive increase in plantaris muscle mass and RNA content derived primarily from myogenic cells. Animals were subjected to synergist ablation (SA) for 3, 7, or 14 d and injected with 2 mg of EU 5 h before being killed. This paradigm enriched for nascent RNA (EU-RNA) labeling within myogenic cells. (A) Schematic for labeling of nascent RNA after mechanical overload. (B) Plantaris muscle mass normalized to the body weight of the animal. *Significant difference between SA7 and SA14 compared with Sham. †Significant difference between SA14 relative to SA3. (C) RNA abundance within the muscle tissue normalized to tissue weight. *Significant difference compared with Sham. (D) Red arrows indicate myogenic nuclei identified by their location within the laminin stain surrounding the myofiber. White arrow indicates a nonmyogenic nucleus outside the laminin stain of the myofiber. Scale bar, 50 μm. (E) Quantification of the percentage of either myogenic or nonmyogenic nuclei that stain positive for EU-RNA during mechanical overload of skeletal muscle. *Significantly greater percentage of EU-RNA–positive nuclei in myogenic cells than in nonmyogenic cells. (F) Enrichment for short-lived (∼4–6 h) cell type–specific markers. Murf1 (myofiber) was significantly enriched relative to Tcf4 (fibroblast cell), Tie2 (endothelial cell), Pax7 (satellite cell), and Emr1 (macrophage cell). *Significantly greater enrichment than with other cell-specific mRNAs. (G) Enrichment for intermediate-lived (12–18 h) cell type–specific markers. Scgc (myofiber) was significantly enriched relative to Col3a1 (fibroblast cell), CD31 (endothelial cell), and Csf2ra (macrophage cell). *Significantly greater enrichment than with all other markers. †Significant enrichment relative to Col3a1 and CD31. For all analyses, p < 0.05 and n = 5–7 at each time point. All data are presented as mean ± SEM.