Figure 12.
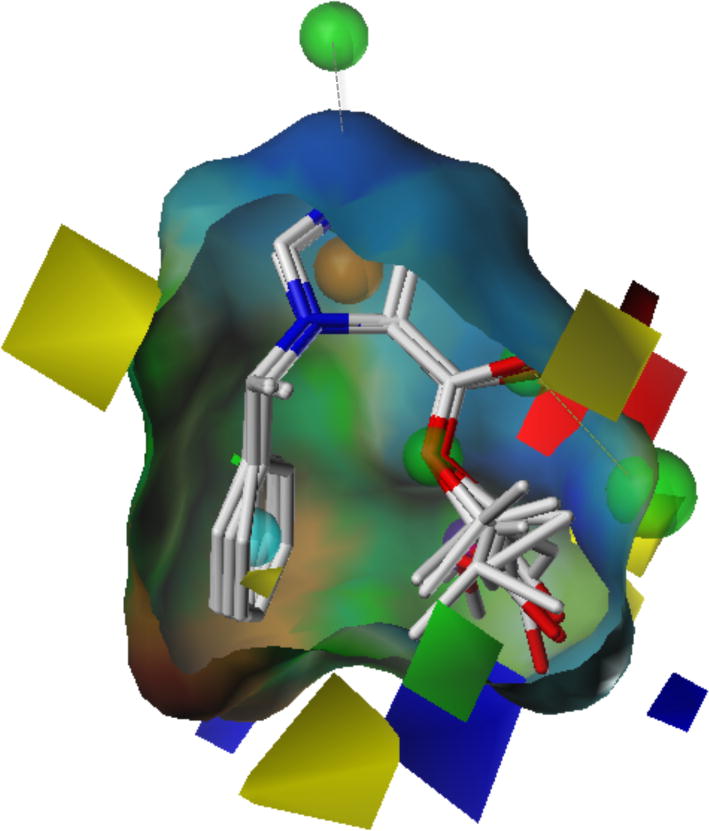
Pharmacophore model of etomidate analogues with the merged surface of the 5 compounds having the highest γ-aminobutyric acid type A (GABAA) receptor potencies. The surface colors are based on lipophilic potential ranging from brown (highly lipophilic) to blue (highly hydrophilic), z-clipping was applied to the surface to match the GABAA receptor comparative molecular field analysis contour maps. The dotted lines show interactions where a pharmacophore point on the ligand could be associated with one on the receptor binding site because the volume constraint of the latter also fell within the 1 Å limit.
