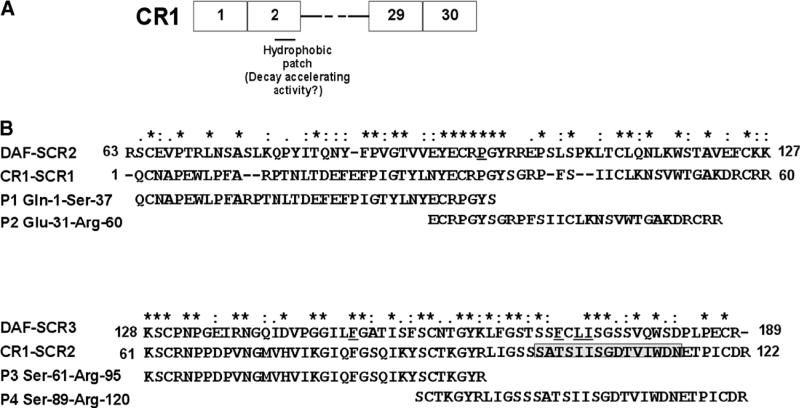
Fig. 2.
CR1 peptides. (A) Schematic representation of CR1 peptides in relation to full-length CR1 is depicted. (B) CLUSTAL W (1.82) alignment of SCR-1 and -2 of CR1 with SCR-2 and -3 of DAF. The degree of conservation is shown above the sequences (*, same residue; “:”, conserved substitution; “.”, semi-conservative substitution). The residues in DAF that have been shown to be critical for decay acceleration of the C3 convertases are underlined. The position of the predicted hydrophobic patch in SCR-2 based on the homology model is shown in boxed grey. The sequence of the peptides 1–4 (P1–4) and their position in relation to full-length SCR-1 and SCR-2 of CR1 are depicted.