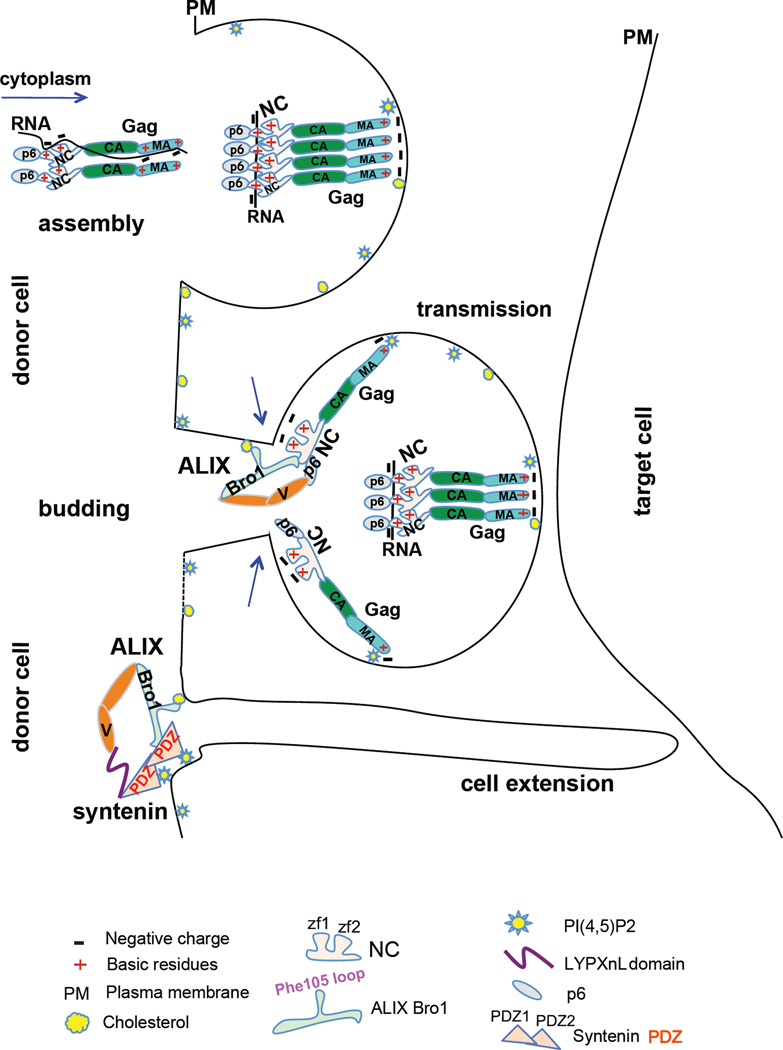
Figure 7.
Striking parallels between NC viral function and syntenin PDZ cellular function. Gag assembly complexes associate with RNA in the cytoplasm, to stimulate Gag nucleation and prevent premature association with internal membranes. At the PM, Gag assembles in specific lipid-enriched (PI(4,5)P2 and cholesterol) domains to favor virus assembly, budding and transmission. Once a spherical particle forms, a subset of NC molecules become positioned closer to the membrane at the top of budding necks and dynamically trade RNA for lipids of the PM, where NC and ALIX Bro1 co-insert in cholesterol-rich domains. This process is known to be transient and precedes ESCRT-III recruitment and catalysis of membrane scission. In the cell, ALIX captures its cellular partner syntenin, whose PDZs associate with PI(4,5)P2-rich domains of the membrane where—like NC—it engages Bro1 to promote important signaling pathways pertinent to health and disease (i.e. cell-to-cell extension)