Supplementary Figure 4.
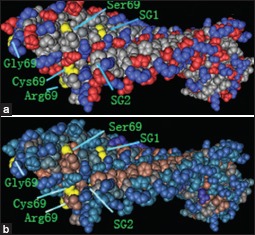
The protein structural charge and hydrophobic domains in the model of dentin matrix protein 1 and outer surface protein A (Protein Data Bank identification code 2FKJ) were visualized using Cn3D software version 4.1 (http://www.ncbi.nlm.nih.gov/Structure/CN3D/cn3d.shtml). The positions of the four amino acid substitutions of rs10019009 associated with ankylosing spondylitis susceptibility in our study are indicated. (a) Structural changes are demonstrated throughout the charged molecule after changes of the amino acid at position 69. (b) Structural changes are shown throughout the hydrophobic domain after changes of the amino acid at position 69. SG1 indicates the position of Ser109Gly110 with the change of serine and cysteine at residue 69. SG2 indicates the position of Ser109Gly110 with the change of arginine and glycine at residue 69.
