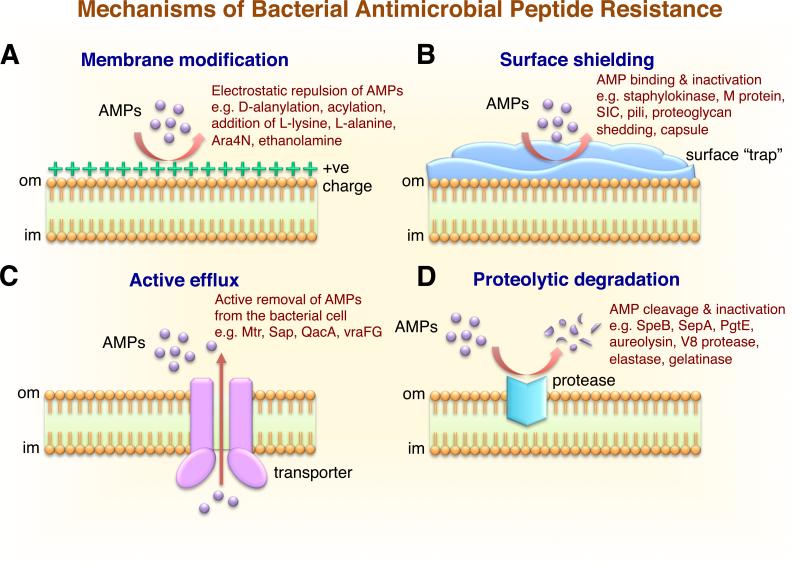
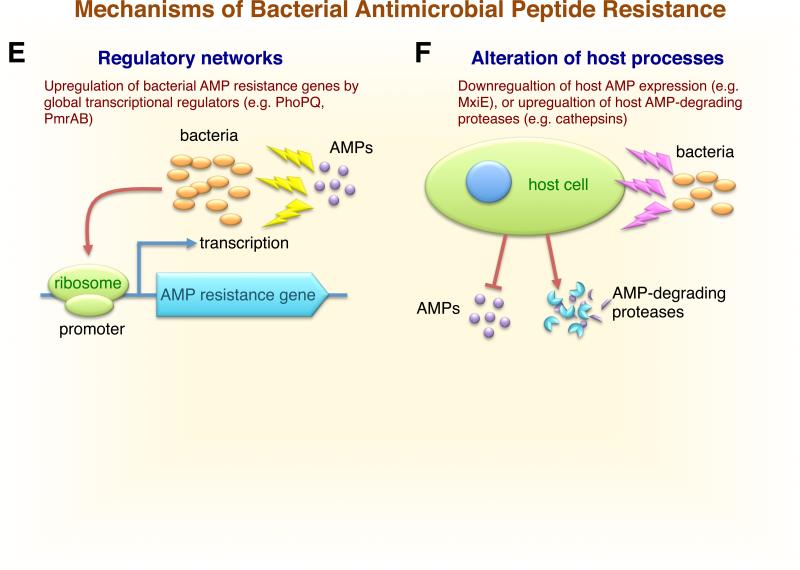
Figure 1.
Schematic representation of the multiple resistance mechanisms developed by bacteria to overcome host antimicrobial peptides. A) Modification of the bacterial outer membrane. Bacterial resistance to cationic antimicrobial peptides is mediated by alterations in surface charge. Gram-positive bacteria: D-alanine modification of cell wall techoic acid (dlt), L-lysine (mprf), or L-alanine modification of phosphatidylglycerol (mprf). Gram-negative bacteria: aminoarabinose or acylation modifications of lipid A in LPS (pmr, pagP), or addition of ethanolamine to lipid A (pmrC, lptA). The increased positive charge on bacterial surface repels cationic AMPs. B) Shielding of the bacterial surface through the trapping and inactivation of AMPs in the extracellular milieu enhances resistance and pathogenicity. Surface-associated capsule traps AMP (e.g. K. pneumoniae cps operon), surface protein binds AMP (e.g. GAS M1 protein, GBS PilB pilus protein), secreted protein binds AMP (e.g. GAS SIC protein or S. aureus staphylokinase), or bacterial proteases release host proteoglycans to block AMP (e.g. P. aeruginosa LasA). C) Membrane efflux pumps function by translocating the AMP out of cell (e.g. Neisseria spp. Mtr, S. Typhimurium Sap, S. aureus QacA, and Staphylococcus spp. VraFG). D) Degradation and inactivation of AMPs by bacterial proteases (e.g. GAS SpeB protease, S. epidermidis SepA, S. Typhimurium PgtE, S. aureus aureolysin and V8 protease, P. aeruginosa elastase, and E. faecalis gelatinase). E) Bacterial exposure to AMPs upregulates the expression of AMP-resistance genes through global gene regulatory networks (e.g. S. Typhimurium and P. aeruginosa PhoPQ and PmrAB). F) Alteration of host processes by bacteria, including the downregulation of host AMP production (e.g. Shigella spp. transcriptional factor MxiE), or the upregulation and activation of host AMP-degrading proteases (e.g. P. aeruginosa). Abbreviations: om, bacterial outer membrane; im, bacterial inner membrane.