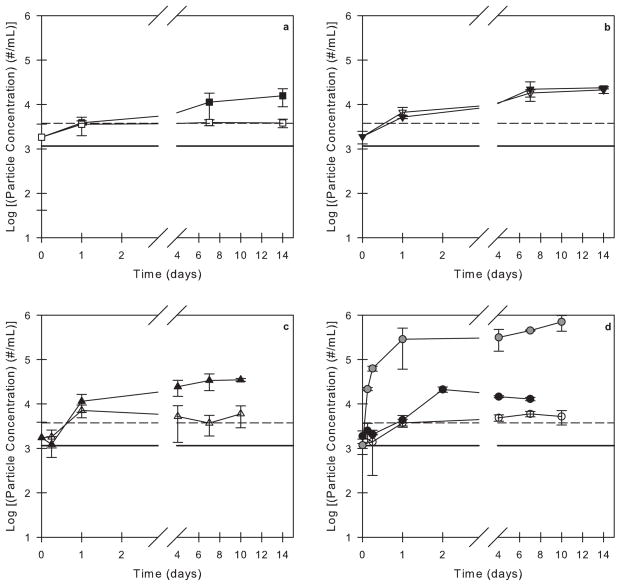
Figure 1.
Particle concentrations in 3M formulations and buffer solutions with 0.01% v/v polysorbate 20 agitated in PFS as a function of time. Open symbols correspond to syringes incubated with no air bubble and closed symbols correspond to syringes incubated with an air bubble. The particle concentrations in a buffer solution (solid black line) and in a 3M solution (dashed black line) with 0.01% v/v polysorbate 20 that were not incubated in syringes are also shown. The incubation conditions are as follows: (a) L-histidine buffer (no protein) in agitated, siliconized syringes, (b) 3M formulation in quiescent, siliconized syringes, (c) 3M formulation in agitated, un-siliconized syringes, and (d) 3M formulation in agitated, siliconized syringes. For comparison, the gray symbols in panel (d) correspond to a 3M formulation with no surfactant agitated in siliconized syringes with an air bubble (data reproduced from Gerhardt et al.36).