Table 1. Electrochemical data measured using cyclic voltammetry (CV) for 3-phenoxymethylmenadione derivatives examined in this work.
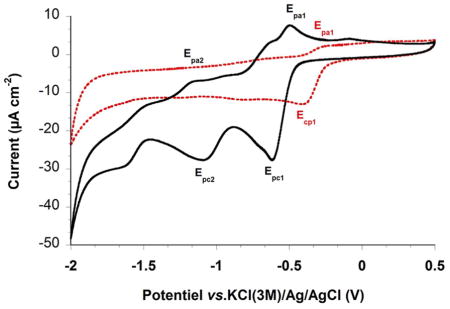
Cyclic voltammograms of 3-phenoxymethylmenadione representatives, the menadione 2 (black bold line) and its difluoromethyl analogue 8 (red dashed line) were recorded at platinum electrode in CHCl2 with 0.1 M Tetra-n-butylammonium tetrafluoroborate (Bu4NBF4) as supporting electrolyte at 200 mV s−1 scan rate.
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Epc1 (V) | Epa1 (V) | Ipc1/Ipa1 | E1½ (V) | ΔEp (mV) | Epc2 (V) | Epa2 (V) | Ipc2/Ipa2 | E2½ (V) | ΔEp (mV) | ΔE½ (mV) | |
| 1 | −0.628 | −0.532 | 1.27 | −0.580 | 96 | −1.265 | irr | irr | irr | irr | NA |
| 2 | −0.620 | −0.492 | 2.29 | −0.556 | 128 | −1.154 | −1.100 | 2 | −1.127 | 54 | 571 |
| 5 | −0.627 | −0.508 | 1.89 | −0.508 | 119 | −1.158 | irr | irr | irr | irr | NA |
| 7 | −0.620 | −0.506 | 2.92 | −0.563 | 114 | −1.132 | −1.120 | 1.75 | −1.126 | 12 | 563 |
| 8 | −0.414 | −0.244 | 3.31 | −0.329 | 170 | ND | ND | ND | ND | ND | ND |
| 9 | −0.326 | −0.215 | 1.67 | −0.271 | 111 | ND | ND | ND | ND | ND | ND |
DMSO;I = 0.1 M n-Bu4NPF6, E½ = (Epc+Epa)/2 (V), ΔE = Epa-Epc (mV), ΔE½ = E1½-E2½ (V)). v = 200 mV s−1; reference electrode = KCl(3 M)/Ag/AgCl; working electrode = glassy carbon disk of 0.07 cm2 area; auxiliary electrode = Pt wire. ND: no second wave observed, irr: irreversible reduction, NA: not applicable.
