Abstract
Scleractinian corals are assumed to be stenohaline osmoconformers, although they are frequently subjected to variations in seawater salinity due to precipitation, freshwater run‐off and other processes. Observed responses to altered salinity levels include differences in photosynthetic performance, respiration and increased bleaching and mortality of the coral host and its algal symbiont, but a study looking at bacterial community changes is lacking. Here, we exposed the coral Fungia granulosa to strongly increased salinity levels in short‐ and long‐term experiments to disentangle temporal and compartment effects of the coral holobiont (i.e. coral host, symbiotic algae and associated bacteria). Our results show a significant reduction in calcification and photosynthesis, but a stable microbiome after short‐term exposure to high‐salinity levels. By comparison, long‐term exposure yielded unchanged photosynthesis levels and visually healthy coral colonies indicating long‐term acclimation to high‐salinity levels that were accompanied by a major coral microbiome restructuring. Importantly, a bacterium in the family Rhodobacteraceae was succeeded by Pseudomonas veronii as the numerically most abundant taxon. Further, taxonomy‐based functional profiling indicates a shift in the bacterial community towards increased osmolyte production, sulphur oxidation and nitrogen fixation. Our study highlights that bacterial community composition in corals can change within days to weeks under altered environmental conditions, where shifts in the microbiome may enable adjustment of the coral to a more advantageous holobiont composition.
Keywords: bacterial community profiling, coral holobiont, coral reef, Fungia granulosa, microbiome, Red Sea
Introduction
Coral reefs are among the most diverse and productive ecosystems on the planet (Reaka‐Kudla et al. 1996) and provide a wide range of goods and services to approximately 500 million people in more than 100 countries (Wilkinson 2008). Coral reef ecosystems rely on the three‐dimensional carbon skeleton framework built by scleractinian corals. Scleractinian corals are metaorganisms—so‐called coral holobionts—that are composed of the coral host, its dinoflagellate endosymbionts (genus Symbiodinium) and a diverse microbial assemblage consisting of fungi, bacteria, archaea and viruses (microbiome) (Rohwer et al. 2002). The bacterial microbiome has been shown to play important roles in coral health (Rosenberg et al. 2007), immunity (Ritchie 2011), as well as carbon, sulphur and nitrogen cycling (Rohwer et al. 2002; Raina et al. 2009; Lema et al. 2012). Moreover, it has been suggested that microbial assemblages facilitate the acclimation of coral holobionts to new environmental conditions (the probiotic hypothesis, Reshef et al. 2006). However, knowledge about the specific role of the vast majority of microbes, and in particular bacteria, to holobiont function is still limited (Lesser et al. 2004; Barott et al. 2011; Morrow et al. 2012; Bourne & Webster 2013).
Although frequently exposed to changes in seawater salinity, for example due to precipitation, freshwater run‐off, periods of prolonged drought or desalination processes (Chartrand et al. 2009; Lirman & Manzello 2009; Roberts et al. 2010; Edge et al. 2013; Hédouin et al. 2015), scleractinian corals are assumed to be stenohaline osmoconformers with a limited ability to adjust to salinity fluctuations (Hoegh‐Guldberg & Smith 1989; Ferrier‐Pages et al. 1999; Kerswell & Jones 2003; True 2012; Hédouin et al. 2015). Nevertheless, studies show that some coral species are able to tolerate greater salinity fluctuations than others. For instance, Stylophora pistillata showed decreased respiration and photosynthetic rates at minor salinity decreases; whereas Siderastrea radians displayed a high resilience towards salinity changes (Ferrier‐Pages et al. 1999; Lirman & Manzello 2009). Further, Coles (2003) reported high‐salinity tolerance of corals in the Arabian Gulf and Red Sea, with some species surviving salinities of 48–50 practical salinity unit (PSU). While higher animals possess excretory systems to adjust for changes in salinity, most marine invertebrates, including scleractinian corals, are considered osmoconformers (Yancey et al. 2002; Evans 2008). Yet, in sea anemones changes in free amino acid pools are involved in osmoregulation (Shick 1991). Similarly, marine microorganisms (i.e. algae and bacteria) accumulate diverse molecules that serve as osmolytes upon increased salinity exposure (Csonka & Hanson 1991; Mayfield & Gates 2007). However, osmoregulation in the coral‐algal endosymbiosis represents a challenging scenario. The coral animal host has to equilibrate the external osmotic pressure with its intracellular environment, which is determined by its own metabolism and that of its algal symbionts (Mayfield & Gates 2007). In this context, the role of the coral‐associated bacteria is virtually unknown.
To elucidate the role of coral‐associated bacteria to salinity changes, we exposed the coral Fungia granulosa to strongly increased salinities resulting from seawater reverse osmosis (SWRO) desalination concentrate. By collecting data from all holobiont compartments (i.e. coral host, symbiont algae, bacterial microbiome) using a combination of short‐ (4 h) and long‐term (29 days) experimental treatments, we aimed to disentangle compartment‐specific responses and to assess potential adaptation/acclimation processes by characterizing the initial response and long‐term effects on the coral holobiont.
Materials and methods
Study site
We used concentrated salt brine from a SWRO desalination plant located at King Abdullah University of Science and Technology (KAUST, Saudi Arabia) to test the effects of increased salinity to F. granulosa. The SWRO currently discharges an average concentrated salt brine of 41 358 m3/day. The submerged discharge structure is located at 22°17.780N, 39°04.444E at 18 m depth with four discharge screens (1800 × 1000 mm) about 6 m above the seafloor (Fig. S1, Supporting information) (van der Merwe et al. 2014b).
Short‐term hypersalinity treatment
Hypersaline water of 55 PSU was collected directly from the desalination plant discharge (see above). A hose was used to collect the concentrate via SCUBA and pumped into a container on an accompanying boat. The collected water was then transferred into 50‐L opaque plastic bins, and salinity was determined (WTW Cond 3310; WTW, Weilheim, Germany). Ambient water (39 PSU) was collected from the top of the desalination discharge structure. We collected 14 specimens of F. granulosa (6–8 cm) on the same day (March 2014) in about 8 km distance at Fsar reef (22°13.945N, 39°01.783E; 14–18 m). The corals were sampled separately in zip‐lock bags and transferred to 50 L opaque plastic containers filled with ambient reef water (39 PSU) upon return to the boat. Four specimens were immediately rinsed with filtered seawater (FSW; 0.22 μm), wrapped in aluminium foil and flash frozen in liquid nitrogen for further analyses (freshly collected corals). The remaining 10 coral specimens were photo‐acclimated to constant experimental light conditions of 120 μmol/m2/s (measured with DIVING‐PAM, Walz, Effeltrich, Germany) for 30 min at 27 °C. Each coral specimen was measured thrice via pulse‐amplitude modulated fluorometry (PAM; measured with DIVING‐PAM) for photochemical efficiency. Subsequently, 10 specimens were transferred into one 1‐L glass beaker each: five were placed in a 39 PSU ‘control’ bin and five in a 55 PSU ‘high‐salinity’ bin, each holding 50 L of water. Four 50 mL water samples were taken from the control and treatment bin each over a 0.45 μm filter attached to a syringe for total alkalinity (TA) measurements. Oxygen concentration (WTW Multi 3500i, WTW), salinity and temperature (both WTW Cond 3310) were measured within each of the coral beakers prior to incubation start. Incubations were stopped after 4 h beaker by beaker. Again, oxygen and photochemical efficiency were measured, and 50 mL water samples from each beaker for TA measurements were taken in duplicate. Corals were rinsed with FSW, wrapped in aluminium foil and frozen in liquid nitrogen until further analysis.
Long‐term hypersalinity treatment
We selected the KAUST SWRO discharge site (see above) for an in situ transplantation experiment, further described in van der Merwe et al. (2014b). Previous measurements suggested increased salinities also at a greater distance (>15 m) from the discharge structure (van der Merwe et al. 2014a). To cover a range of salinities resulting from the brine discharge and to assess the discharge dilution pattern and the salinity impact on the coral F. granulosa, six stations were chosen along a transect: station 1 in the discharge screen, all other stations on the seafloor at 0, 2.5, 5, 15 and 25 m distance northwards from the discharge structure (Fig. S1, Supporting information). Environmental parameters [i.e. salinity, temperature, dissolved oxygen, photosynthetically active radiation (PAR)] along the transect were assessed at each station (van der Merwe et al. 2014b). For the long‐term experiment, 18 specimens of F. granulosa were collected from 14 to 18 m depth at Fsar reef (January 2014). Collected corals were acclimated at ambient salinity on the desalination discharge structure roof for 20 h and tagged with nylon fishing line and labels. Three corals were randomly distributed to each of the six stations. The corals were fixed onto bricks (stations 2–6) and to the screen grid (station 1), respectively (van der Merwe et al. 2014b). All corals were measured thrice via PAM 1 h after attachment and visually assessed for any signs of bleaching; additionally water samples (1‐L cubitainer) and PAR measurements were taken at each station. To test for the degree of correlation between changes in ambient light levels and effective quantum yield, we used sigmaplot 11 (SYSTAT Software, Point Richmond, CA, USA) to conduct a linear regression analysis. All water samples were measured for dissolved oxygen and salinity immediately upon return to the boat. The sampling procedure was repeated after 1, 4, 6, 8, 15 and 29 days and routinely took place between 11:00 h and 12:00 h. After 29 days, water samples from each station were taken for assessment of reef water bacterial community. All coral specimens were collected into separate zip‐lock bags, rinsed with FSW on board, wrapped in aluminium foil and flash frozen in liquid nitrogen until further analysis.
Coral physiology
We determined coral calcification rate (G) according to the total alkalinity (TA) anomaly method (Schneider & Erez 2006). Each 50 mL water sample was analysed for TA using an automated titrator (Titrando 888; Metrohm AG, Herisau, Switzerland) with 0.01 m HCl. Following the Gran approximation, we used the second endpoint of the titration curve to estimate TA (Grasshoff et al. 2009). We used the difference in TA between initial and final sample, normalized over incubation time (T) and coral tissue surface area (SA) to calculate G as follows (Schneider & Erez 2006):
We used the difference of dissolved oxygen concentration at incubation start and end, and normalized over T and SA to calculated net photosynthesis (P n) (Schneider & Erez 2006):
Corals were modelled as cylinders as this approximates their shape well; diameter and height were measured using a calliper. Coral volume (V) and surface area (A) were calculated following: Surface area A = πr2, Volume V = πhr2.
Additionally, we assessed the photochemical efficiency of F. granulosa via PAM fluorometry (DIVING‐PAM). The effective quantum yield (Genty et al. 1989):
reflects the efficiency of photosystem II (PSII) under ambient light adapted conditions (Ralph & Gademann 2005). Ambient light conditions were logged with the PAM's fibre quantum sensor. The light intensity for the incubation experiment (about 120 μmol/m2/s) represents roughly the measured light intensity at the collection site (15 m; 100–150 μmol/m2/s).
16S rRNA gene sequencing
Flash frozen corals were stored at −80 °C until DNA extraction. Each specimen was carefully unwrapped on ice, transferred into a sterile zip‐lock bag and doused with 5 mL Qiagen RLT buffer (Qiagen AllPrep kit; Hilden, Germany). While thawing, buffer and coral tissue were carefully blasted off using tap air pressure and pipette barrier tips. The buffer–tissue mixture was transferred into 15‐mL Falcon tubes and vortexed. A 500 μL aliquot was used for DNA extraction following the manufacturer's protocol (Qiagen AllPrep kit). DNA concentrations were quantified on a NanoDrop 2000C spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Water samples were transported to the laboratory on ice in the dark. We filtered 1 L of each water sample over 0.22 μm Durapore PVDF filters (Millipore, Billerica, MA, USA). Filters were frozen at −80 °C. Half of each filter was cut into small stripes with sterile razorblades and transferred into 2‐mL Eppendorf vials. After adding 400 μL Qiagen RLT buffer, the samples were incubated on a rotating wheel for 20 min. Subsequent extraction steps were conducted following the manufacturer's protocol (Qiagen AllPrep kit).
For PCR amplification, we used about 60 ng DNA for coral samples and about 5 ng DNA for water samples. We used the primers 784F [5′‐TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGAGGATTAGATACCCTGGTA‐3′] and 1061R (5′‐ GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGCRRCACGAGCTGACGAC‐3′) that target variable regions 5 and 6 of the 16S rRNA gene (Andersson et al. 2008) and have been shown to amplify well with coral DNA (Bayer et al. 2013). The primers contain Illumina adapter overhangs (underlined above; Illumina, San Diego, CA, USA). All PCRs were performed in triplicates using Qiagen Multiplex PCR kit with 0.2 μm of each primer adjusted to a total volume of 20 μL with RNase‐free water. The amplification cycling temperatures were set to one cycle at 95 °C for 15 min, 25 cycles each at 95 °C for 30 s, 55 °C for 90 s and 72 °C for 30 s; a final extension step at 72 °C for 10 min. Ten microlitres of each sample was used for visual quality check via 1% agarose gel electrophoresis. The triplicate PCRs of each sample were then pooled and cleaned with Agencourt AMPure XP magnetic bead system (Beckman Coulter, Brea, CA, USA). The clean PCR product then underwent an indexing PCR to add Nextera XT indexing and sequencing adapters (Illumina) according to the manufacturer's protocol. Indexed PCR products were cleaned up using the Agencourt AMPure XP protocol, quantified on the BioAnalyzer (Agilent Technologies, Santa Clara, CA, USA) and QuBit (Quant‐IT dsDNA Broad Range Assay Kit; Invitrogen, Carlsbad, CA, USA), and pooled in equimolar ratios. The final pooled library was purified on a 2% agarose gel to remove any excess primer dimers. The library was sequenced at 8 pm with 10% phiX on the Illumina MiSeq, 2 × 300 bp paired‐end version 3 chemistry according to the manufacturer's specifications. Sequences determined in this study have been deposited in the NCBI Sequence Read Archive under accession no. PRJNA282461 (http://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA282461).
Bacterial community analysis
For amplicon analysis, we used mothur (http://www.mothur.org/ version 1.16.1, Schloss et al. 2009). Sequence reads were split according to barcodes, assembled to contigs and quality trimmed. Duplicates, that is identical sequences, were merged using the ‘unique.seqs’ command to save computation time, and the command ‘count.seqs’ was used to keep a count of the number of sequences over samples represented by the remaining representative sequence. Rare sequence reads were removed (n < 10 over all samples), remaining sequences were aligned against silva (release 119, Pruesse et al. 2007), and a preclustering step (2 bp difference) was performed (Huse et al. 2010). Chimeric sequences were removed using UCHIME as implemented in mothur (Edgar et al. 2011). Additionally, chloroplasts, mitochondria, archaea, eukaryotes and unknown reads were removed. Sequences were classified against Greengenes (McDonald et al. 2012) using a bootstrap of 60, and the sample compositions were compared on a family level. For further analyses, sequences were subsampled to 10 000 sequence reads and a 97% similarity cut‐off level was chosen to obtain OTUs. Chao1 (Chao 1984), Simpson evenness, Inverse Simpson Index, principal coordinate analysis (PCoA) and analysis of molecular variance (amova; Excoffier et al. 1992) were performed as implemented in mothur. PCoA results were plotted in sigmaplot 11. To identify the main contributing OTU for similarity within each of the groups (i.e. freshly collected coral, short‐term ambient salinity, short‐term high salinity, long‐term ambient salinity and long‐term high salinity), a similarity percentages (SIMPER) analysis was performed using primer v6 software (Clarke & Gorley 2006). To identify OTUs that were significantly different between long‐term hypersaline conditions and all other treatments, we used the statistical package indicspecies (Cáceres & Legendre 2009) in r (R Core Team 2014). We employed the BEST (BIO‐ENV) routine in primer to identify the ‘best’ match between OTU distributions and associated environmental variables (i.e. salinity, temperature, dissolved oxygen, light levels, effective quantum yield and depth). OTU abundances and environmental data were square‐root‐transformed and environmental data were normalized. Bray–Curtis similarity was used to calculate an OTU resemblance matrix, and Euclidean distance was used for the environmental parameter resemblance matrix. The weighted Spearman rank‐correlation method was used to identify the parameter (or combination of parameters) providing the highest ρ.
To assess putative functional profiles based on the 16S community composition, we used metagenassist for automated taxonomic‐to‐phenotypic mapping (Arndt et al. 2012). Input files were created in mothur using the ‘make.shared’ and ‘classify.otu’ commands based on all coral samples. During data processing in metagenassist, all 2152 distinct OTUs were assigned, mapped and condensed into 400 functional taxa and filtered based on interquantile range (Hackstadt & Hess 2009). After filtering, 360 functional taxa remained and were normalized over samples by sum and over taxa by range scaling. We then analysed the data for ‘metabolism by phenotype’ and used Euclidean distance measure and average clustering algorithm to visualize the results in a heatmap. To confirm patterns obtained by metagenassist, we investigated specific genes associated with identified processes via picrust (Langille et al. 2013). Input files for picrust were created in mothur using the ‘make.biom’ command including all coral samples. Clusters of orthologous groups were created by predicting the metagenomes in picrust.
Results
Coral and Symbiodinium physiology after short‐term hypersalinity treatment
To assess short‐term effects of strongly increased salinity on coral holobiont function, we determined physiological parameters from the coral host and Symbiodinium at the end of 4‐h incubations in ambient (39 PSU) and hypersaline treatments (55 PSU) (Table 1). We could not visually detect any signs of bleaching (Table 1), but we observed increased mucus production including small bubbles in the high‐salinity treatments (Fig. S2, Supporting information). Corals displayed an about eightfold decreased calcification rate (G) under hypersaline compared to ambient conditions (0.031 ± 0.073 compared to 0.243 ± 0103 CaCO3 μmol/cm2/h, P t‐test < 0.05). Oxygen net production (i.e. oxygen‐producing photosynthesis mainly by Symbiodinium, respiration mainly by coral host and Symbiodinium, bacterial contribution to both) was higher under ambient (start: 8.03 ± 0.05 mg/L; stop: 10.47 ± 1.15 mg/L) than under hypersaline (start: 7.14 ± 0.11 mg/L; stop: 8.44 ± 0.45 mg/L) conditions. Net photosynthesis‐based oxygen production (P n) was significantly higher in ambient condition (13.49 ± 4.87 compared to 8.28 ± 3.98 μg O2/cm2/h, P t‐test < 0.05). As expected, the effective quantum yield (ϕPSII) of PSII in Symbiodinium showed no distinct differences between corals from ambient‐ and high‐salinity conditions at experiment start (0.709 ± 0.019 and 0.710 ± 0.019). In contrast, ϕPSII was significantly decreased for the hypersalinity treatment after the 4‐h incubation period (0.681 ± 0.018 compared to 0.718 ± 0.018 under ambient conditions, P t‐test < 0.05).
Table 1.
Physiological response of coral host and algal symbiont to short‐ and long‐term hypersalinity exposure
| Short‐term (4 h) | Long‐term (29 days) | |||
|---|---|---|---|---|
| 39 PSU | 55 PSU | ~41 PSU | ~49 PSU | |
| Calcification rate (CaCO3 μmol/cm2/h) | 0.243 ± 0.103 | 0.031 ± 0.073* | NA | NA |
| Photosynthetic efficiency (ϕPSII) | 0.718 ± 0.018 | 0.681 ± 0.018* | 0.690 ± 0.015 | 0.705 ± 0.009 |
| Coral tissue discoloration (bleaching) | Not visually apparent | Not visually apparent | Not visually apparent | Not visually apparent |
*Pt‐test < 0.05; NA = not available, values are shown as means ± SD.
Coral and Symbiodinium physiology after long‐term hypersalinity treatment
Fungia granulosa specimens showed no signs of impaired photosynthetic performance during and after a 29‐day in situ experiment (van der Merwe et al. 2014b). We found a rapid dilution of the discharged brine: Only salinity levels at station 1 (i.e. at the SWRO discharge screen) were increased (average 49.4 ± 2.0 PSU) and significantly (P ANOVA < 0.05) different from all other stations (2–6), where salinity was on average 41.3 ± 0.7 PSU and not significantly different between stations (P ANOVA > 0.05). Therefore, corals from station 1 were assigned to the hypersalinity long‐term treatment (n = 3) and corals from all other stations were pooled into the long‐term ambient group (n = 15). Water temperature decreased slightly with increasing distance from the discharge, but differences did not exceed 0.5 °C across average temperatures (stations 1–6, AVG: 26.3 °C, 26.1 °C, 26.0 °C, 26.0 °C, 25.8 °C and 25.8 °C). Dissolved oxygen varied between 5.75 and 6.37 mg/L and was not significantly different (P ANOVA > 0.05) between stations. Symbiodinium effective quantum yields (ϕPSII) were stable for all corals regardless of high‐ or ambient‐salinity conditions. Average ϕPSIIs were within the natural range measured at the collection site (0.682–0.709) with 0.705 ± 0.009 (station 1), 0.694 ± 0.008 (station 2), 0.687 ± 0.007 (station 3), 0.687 ± 0.019 (station 4), 0.693 ± 0.017 (station 5) and 0.690 ± 0.02 (station 6). Differences across the stations—and between daily measurements—coincided with light levels as assessed by PAR values (van der Merwe et al. 2014b), as shown by a linear regression analysis (R 2 = 0.80, P < 0.001). ϕPSII measurements from station 1, reflecting ambient levels from the collection site, indicate that high salinity did not impair the photosynthetical efficiency of Symbiodinium in hospite.
Seawater and coral bacterial communities
We produced 41 16S rRNA gene libraries with a total of 4 784 475 reads distributed over nine water samples, four freshly collected F. granulosa colonies, 10 short‐term incubation corals (five high and five ambient salinity) and 18 long‐term treatment corals (three high salinity at station 1; 15 ambient, i.e. three from each of stations 2–6). After quality trimming, chimera detection and removal of undesired (e.g. chloroplasts) and rare sequences (n < 10 over all samples), 2 612 132 reads with an average length of 292 bp were retained for further analyses. To assess sample‐ and condition‐specific differences in bacterial composition, we classified all sequences to the family level (Fig. 1). Water samples were dominated by Pelagibacteriaceae (~60–70%) and markedly different from all coral samples, but similar to each other. Coral samples were dominated by bacteria from the family Rhodobacteraceae (~20–80%), except for long‐term hypersalinity coral samples, which appeared more even and were not dominated by a distinct bacterial family (Fig. 1).
Figure 1.
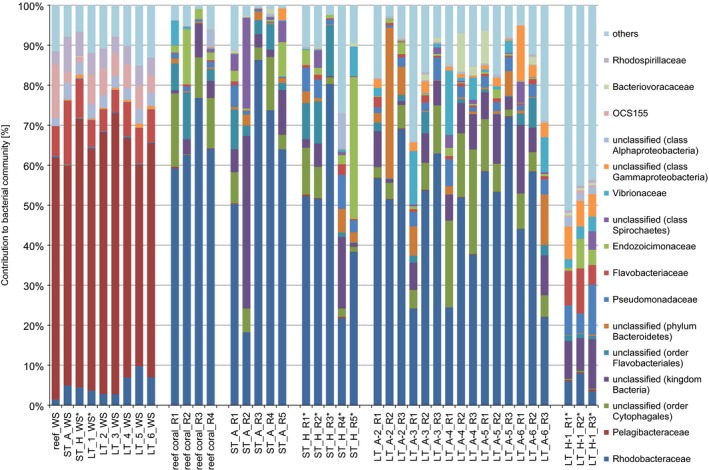
Bacterial taxonomy stack plot on the phylogenetic level of family (greengenes database, bootstrap ≥60). Each colour represents one of the 16 most abundant families in all samples. All other taxa are grouped under category ‘others’. ST, short‐term incubation; LT, long‐term treatment; A, ambient; H, hypersaline; numbers in the LT sample names denote transect station; WS, water sample; asterisks denote hpersaline samples.
Coral microbiome restructuring after long‐term salinity exposure
To reveal differences in bacterial community composition between treatments and over time, we subsampled to 10 000 reads and clustered sequence data to operational taxonomic units (OTUs, similarity cut‐off ≤ 0.03). Good's estimator (Good 1953) ranged between 0.98 and 1.0 coverage for all samples indicating that the majority of bacterial diversity was represented. Chao1 estimates of species richness, Simpson evenness, and Inverse Simpson Index (diversity measure) were all highest for long‐term hypersalinity samples compared to all other samples—with an approximate average 10‐fold increase for diversity (Inverse Simpson Index) and a threefold increase for Chao1 and Simpson evenness (Table 2). We compared microbial community abundance profiles over coral colonies, treatments and time points in a PCoA based on Bray–Curtis dissimilarity (Fig. 2). Water samples were clustering closely together and differed significantly from all coral samples (pamova ≤ 0.005), but not between each other (pamova = 1) confirming that bacterial communities from hypersaline water samples did not exhibit differences in comparison with ambient water samples (Fig. S3, Supporting information). Importantly, we observed a distinct grouping among coral samples. Long‐term hypersaline coral samples clustered significantly away from all other coral samples (i.e. freshly collected, short‐term incubations and long‐term ambient) (pamova ≤ 0.001), and all other coral samples were not significantly different from each other (pamova ≥ 0.05). To uncover the main environmental driver underlying the coral microbiome changes, we employed a biological–environmental matching routine that compares OTU distribution to environmental parameters. This analysis revealed a significant correlation between environmental data (i.e. salinity, temperature, dissolved oxygen, light levels, effective quantum yield and depth) and the coral microbiomes. From all considered environmental parameters and combinations thereof, salinity as a single factor best explained variations in OTU abundance and distribution (ρ = 0.639, PBEST ≤ 0.01) (Table S1, Supporting information).
Table 2.
Summary statistics of 16S rRNA gene amplicon sequencing detailing reef water and microbial communities associated with Fungia granulosa under ambient and hypersaline conditions
| Group | Sample | Treatment | No. of reads | Coverage* | No. of OTUs* | Chao1* | Simpson evenness* | Inverse Simpson Index* |
|---|---|---|---|---|---|---|---|---|
| Reef water | reef_WS | — | 41 689 | 1.00 | 136 | 195 | 0.026 | 3.540 |
| ST_A_WS | 39 PSU | 47 773 | 1.00 | 132 | 159 | 0.033 | 4.364 | |
| ST_H_WS | 55 PSU | 37 985 | 0.99 | 181 | 280 | 0.016 | 2.920 | |
| LT_1_WS | ~49 PSU | 42 098 | 0.99 | 185 | 266 | 0.018 | 3.373 | |
| LT_2_WS | ~41 PSU | 68 342 | 1.00 | 158 | 217 | 0.019 | 2.968 | |
| LT_3_WS | ~41 PSU | 49 940 | 0.99 | 158 | 236 | 0.016 | 2.546 | |
| LT_4_WS | ~41 PSU | 41 004 | 1.00 | 171 | 213 | 0.021 | 3.524 | |
| LT_5_WS | ~41 PSU | 44 270 | 0.99 | 177 | 234 | 0.026 | 4.560 | |
| LT_6_WS | ~41 PSU | 37 508 | 1.00 | 158 | 186 | 0.023 | 3.622 | |
| Freshly collected corals | reef coral_R1 | — | 105 511 | 1.00 | 129 | 161 | 0.021 | 2.646 |
| reef coral_R2 | — | 29 560 | 1.00 | 98 | 119 | 0.025 | 2.408 | |
| reef coral_R3 | — | 20 358 | 1.00 | 61 | 80 | 0.027 | 1.661 | |
| reef coral_R4 | — | 158 984 | 0.99 | 168 | 260 | 0.014 | 2.343 | |
| Short‐term ambient salinity | ST_A_R1 | 4 h at 39 PSU | 92 228 | 0.99 | 206 | 237 | 0.019 | 3.984 |
| ST_A_R2 | 132 436 | 1.00 | 98 | 129 | 0.040 | 3.939 | ||
| ST_A_R3 | 65 695 | 1.00 | 64 | 106 | 0.021 | 1.370 | ||
| ST_A_R4 | 99 762 | 1.00 | 78 | 112 | 0.023 | 1.808 | ||
| ST_A_R5 | 51 040 | 1.00 | 84 | 111 | 0.033 | 2.814 | ||
| Short‐term hypersaline | ST_H_R1 | 4 h at 55 PSU | 51 437 | 1.00 | 113 | 118 | 0.041 | 4.685 |
| ST_H_R2 | 69 779 | 0.99 | 174 | 223 | 0.022 | 3.809 | ||
| ST_H_R3 | 76 435 | 1.00 | 69 | 96 | 0.022 | 1.534 | ||
| ST_H_R4 | 10 393 | 1.00 | 139 | 149 | 0.077 | 10.670 | ||
| ST_H_R5 | 55 964 | 1.00 | 110 | 125 | 0.037 | 4.050 | ||
| Long‐term ambient salinity | LT_A_2_R1 | 29 days at ~41 PSU | 37 060 | 0.99 | 348 | 525 | 0.009 | 3.291 |
| LT_A_2_R2 | 77 465 | 0.99 | 140 | 227 | 0.019 | 2.688 | ||
| LT_A_2_R3 | 75 949 | 0.99 | 239 | 300 | 0.009 | 2.068 | ||
| LT_A_3_R1 | 39 101 | 0.99 | 295 | 376 | 0.035 | 10.420 | ||
| LT_A_3_R2 | 15 655 | 0.99 | 282 | 334 | 0.015 | 4.188 | ||
| LT_A_3_R3 | 59 872 | 1.00 | 141 | 184 | 0.017 | 2.391 | ||
| LT_A_4_R1 | 63 348 | 0.99 | 272 | 314 | 0.033 | 9.006 | ||
| LT_A_4_R2 | 123 875 | 0.99 | 310 | 495 | 0.011 | 3.317 | ||
| LT_A_4_R3 | 72 038 | 0.99 | 276 | 399 | 0.017 | 4.752 | ||
| LT_A_5_R1 | 68 602 | 0.99 | 264 | 371 | 0.011 | 2.823 | ||
| LT_A_5_R2 | 106 800 | 0.98 | 446 | 631 | 0.009 | 4.147 | ||
| LT_A_5_R3 | 55 622 | 0.99 | 228 | 279 | 0.008 | 1.903 | ||
| LT_A_6_R1 | 121 617 | 0.99 | 228 | 377 | 0.019 | 4.338 | ||
| LT_A_6_R2 | 47 763 | 0.99 | 265 | 327 | 0.013 | 3.565 | ||
| LT_A_6_R3 | 64 844 | 0.99 | 328 | 424 | 0.042 | 13.754 | ||
| Long‐term hypersaline | LT_H_1_R1 | 29 days at ~49 PSU | 32 455 | 0.99 | 608 | 685 | 0.091 | 55.242 |
| LT_H_1_R2 | 63 823 | 0.98 | 736 | 835 | 0.076 | 56.261 | ||
| LT_H_1_R3 | 56 052 | 0.98 | 864 | 968 | 0.042 | 36.288 |
*, subsampled to n = 10000; ST, short‐term incubation; LT, long‐term treatment; A, ambient; H, hypersaline; Numbers in the LT sample names denote transect station; WS, water sample.
Total number of OTUs = 2235.
Figure 2.
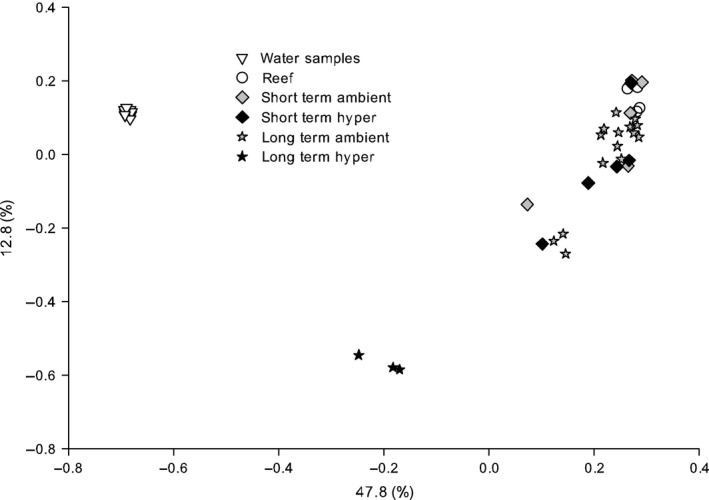
Clustering of coral samples based on Bray–Curtis dissimilarity of microbial community abundances in a principal coordinate analysis (PCoA) (R 2 = 0.91). Water samples = reef water, reef = freshly collected Fungia granulosa, short‐term ambient = 4 h at 39 PSU, short‐term hyper = 4 h at 55 PSU, long‐term ambient = 29 days at 41 PSU, long‐term hyper = 29 days at 49 PSU, percentages on axes indicate variation explained by the two coordinates.
To assess overall similarity and to identify the main contributing OTU within each of the groups (i.e. freshly collected coral, short‐term ambient salinity, short‐term hypersalinity, long‐term ambient salinity and long‐term hypersalinity), we tested for the presence of ‘core’ microbiome OTUs with SIMPER (similarity percentages) analysis. All groups showed a consistent ‘within‐group’ similarity of about 50% (Bray–Curtis) (Table 3). Looking at the main contributing OTU within long‐term hypersalinity samples, we identified OTU0010 (Pseudomonas veronii) (Table 3). The average abundance of this OTU was increased by about threefold in corals from the long‐term hypersaline treatment compared to all other coral samples (836.7 vs. 270.7 average read counts) (Table S2, Supporting information). In contrast, corals from all other groups revealed the same single, unclassified OTU in the family Rhodobacteraceae (OTU0001) to be the numerically abundant bacterial taxon (Table 3). The average abundance of OTU0001 for these groups was 5105.6 reads (relative abundance 51.1%) compared to an average abundance of only 133 reads (relative abundance 1.33%) of this OTU in long‐term hypersalinity coral samples (Table 3; Table S2, Supporting information).
Table 3.
Summary of SIMPER analyses showing the main contributing OTU in each treatment group. Displayed are Bray–Curtis similarity measures between group members based on OTU abundances within a sampling group, the main contributing OTU, the average abundance of this OTU and the average contribution of this OTU to the overall group similarity
| Treatment group (Bray–Curtis similarity) | Main contributing OTU (bootstrap value) | AVG read abundance | AVG contribution (%) |
|---|---|---|---|
| Freshly collected coral (51.19%) | OTU0001 unclassified sp.(100), family Rhodobacteraceae(100) | 6530 | 36.53 |
| Short‐term ambient (49.98%) | OTU0001 unclassified sp.(100), family Rhodobacteraceae(100) | 5627.2 | 30.65 |
| Short‐term hypersalinity (47.95%) | OTU0001 unclassified sp.(100), family Rhodobacteraceae(100) | 4594.4 | 23.42 |
| Long‐term ambient (44.03%) | OTU0001 unclassified sp.(100), family Rhodobacteraceae(100) | 4722.3 | 19.29 |
| Long‐term hypersalinity (56.20%) | OTU0010 Pseudomonas veronii(93), family Pseudomonadaceae(100) | 836.7 | 2.22 |
Based on the similarity of diversity estimates, proximity in PCoA clustering and an identical main contributing OTU for the microbial communities of freshly collected coral, short‐term ambient salinity, short‐term hyper‐salinity and long‐term ambient salinity treatments, we jointly compared corals from these treatments to corals from the long‐term hyper‐salinity treatment. Differentially abundant OTUs were determined with indicspecies and revealed that 523 OTUs were significantly (P ≤ 0.01) different between corals from the hypersalinity long‐term treatment in comparison to all other coral samples. Interestingly, only 3 OTUs (of a total of 4 OTUs) were highly significantly (P ≤ 0.001) enriched in all coral groups, but absent in corals from the hypersalinity long‐term treatment. These were OTU0003, OTU0005 (both unclassified spp., order Cytophagales) and OTU0009 (unclassified sp., family Rhodobacteraceae) (Table S3, Supporting information). In contrast, indicspecies analysis identified 519 significantly enriched OTUs in corals from the hypersalinity long‐term treatment, which together made up a relative abundance of 55.6%. Of those, 5 OTUs had an average abundance of at least 100 reads and 104 OTUs had an average abundance of at least 10 reads and were highly significantly enriched in corals from the hypersalinity long‐term treatment (P ≤ 0.001; Table S3, Supporting information). The highly significant enrichment of hundreds of OTUs under hypersalinity conditions over a course of 29 days in connection with the parallel decrease of the otherwise numerically abundant OTU (i.e. OTU0001) demonstrate a major restructuring of the coral microbiome. In contrast to previous findings (Bouvier & del Giorgio 2002), bacterial communities in the seawater did not change upon high‐salinity exposure, presumably because of a rapid brine dilution (van der Merwe et al. 2014b) and thus short residence times of associated bacteria therein.
Taxonomy‐based functional profiling of bacterial communities
We used metagenassist to predict putative changes in bacterial community function based on differences in bacterial community composition. In line with our taxonomy‐based analysis, the long‐term hypersalinity samples formed a distinct group from all other coral samples (Fig. 3). In these three samples, we found a pronounced upregulation of ‘polyhydroxybutyrate (PHB) storage’, ‘sulphur oxidizer’, ‘syntrophy’, as well as ‘xylan’, ‘alkane’ and ‘biomass degraders’ (Fig. 3). At the same time, ‘dehalogenation’, ‘ammonia oxidizer’ as well as ‘nitrite’ and ‘sulphate reducer’ were downregulated. Next, we used picrust to analyse specific genes underlying identified processes (i.e. PHB metabolism, sulphur cycling and nitrogen cycling) affected in the long‐term hypersaline samples. Further supporting the results from metagenassist, we found decreased abundance of the enzyme PHB depolymerase associated with PHB metabolism, decreased anaerobic DMSO reductase and sulphite reductase associated with sulphur cycling and decreased nitrite reductase associated with the process of nitrite reduction (Fig. S4, Supporting information). Besides these processes, some samples were enriched for other functions, for example ‘iron oxidizer’, ‘sugars fermentor’, ‘chitin degradation’, ‘selenate reducer’ (among others) in metagenassist. However, samples enriched for these functions did not reveal any apparent patterns in regard to treatment (i.e. control, ambient or hypersalinity) or exposure time (i.e. 4 h or 29 days).
Figure 3.
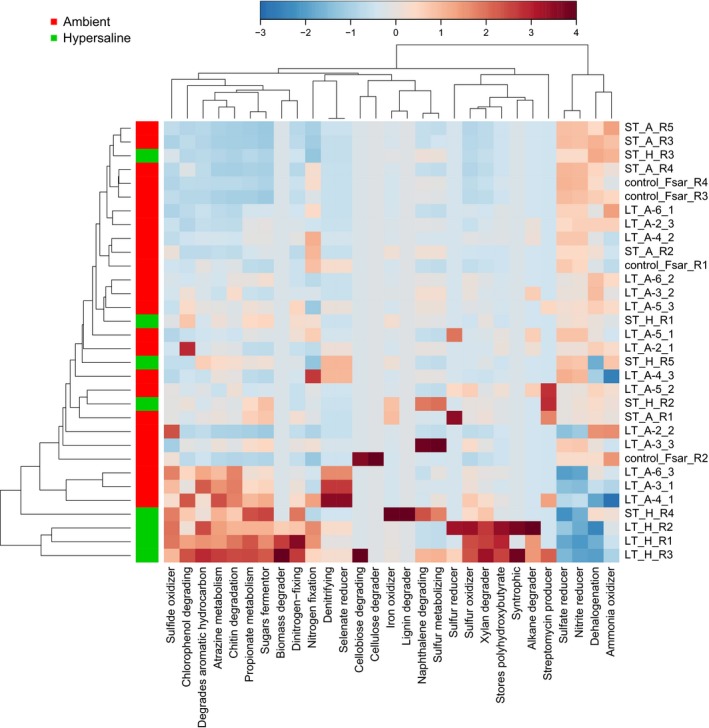
Taxonomy‐based functional profiling of bacterial communities. Heatmap created in metagenassist displaying changes in putative functional profiles based on the 16S community composition. Changes are displayed on a relative scale with enrichment in red and depletion in blue. Data were analysed for metabolism by phenotype with an Euclidean distance measure and average clustering algorithm. ST, short‐term incubation; LT, long‐term treatment; A, ambient; H, hypersaline; Numbers in the LT sample names denote transect station.
Discussion
In this study, we assessed the effect of short‐ and long‐term hypersalinity exposure on the Red Sea coral F. granulosa. Our results indicate distinct short‐ and long‐term reactions of the coral holobiont. The short‐term experiment, aimed to measure the initial response, was characterized by an absence of changes in the bacterial community structure, but a significant reduced calcification and photosynthesis under strongly increased salinity levels. In contrast, the long‐term transect experiment indicated a putative acclimation response, since corals exposed to high salinity for 29 days did not exhibit measureable (photo)physiological effects or signs of bleaching (van der Merwe et al. 2014b), but rather, displayed a significant shift in the associated bacterial community.
Short‐term and long‐term coral physiology
Fungia granulosa is a single‐polyp scleractinian coral that has been demonstrated to possess a slow growth rate (Chadwick‐Furman et al. 2000). Accordingly, our values for the calcification rate (G) were lower than those reported for other corals, for example for Stylophora subseriata (1.05–1.73 μmol CaCO3/cm2/h) (Sawall et al. 2011). It is of note that calcification effectively stopped at high salinities in the short‐term treatment. Unfortunately, calcification rates are not available for the long‐term treatment. However, it would be interesting to see whether calcification rates are not influenced in long‐term hypersaline exposure—as observed for photosynthetic efficiency.
In line with previous studies, our short‐term incubation showed an overall oxygen increase, which was significantly lower for high‐salinity conditions (Gattuso et al. 1999; Manzello & Lirman 2003; Lirman & Manzello 2009). Further, a reduction of photosynthetic rates has been documented for hyper‐ and hyposaline scenarios before (Muthiga & Szmant 1987; Moberg et al. 1997; Ferrier‐Pages et al. 1999; Alutoin et al. 2001; Chartrand et al. 2009). However, long‐term ϕPSII values from our coral samples were in the same range as photosynthetic yields originating from corals in their natural environment. We could not observe any differences between hypersaline and ambient conditions in regard to the corals' photophysiology or bleaching status (van der Merwe et al. 2014b).
In accordance with data collected from our short‐ and long‐term experiments, an initial sharp decline in photosynthetic performance with subsequent recovery has been suggested as an acclimation pattern (Manzello & Lirman 2003; Lirman & Manzello 2009). Mayfield & Gates (2007) interpreted these patterns as an indication for osmoregulatory processes, also considering corals to generally tolerate slow salinity changes better than more rapid ones (Muthiga & Szmant 1987). Our physiological data are supporting corals as being able to adjust to salinity. We report distinct physiological effects on coral host (calcification) and Symbiodinium (decreased photosynthesis) in our 4‐h incubation. In the long‐term in situ experiment, we could not find any influence on the (photo)physiology of F. granulosa indicating acclimation of the coral holobiont to prevailing salinity levels.
Coral microbiome restructuring
To our knowledge, this is the first study that assessed coral bacterial microbiome structure under short‐ and long‐term exposure of corals to salinity changes. Healthy corals maintain mostly specific, stable and uneven microbial assemblages indicating selected microbiomes (Bourne et al. 2008; Meron et al. 2011a, 2012; Bayer et al. 2013; Bourne & Webster 2013; Jessen et al. 2013; Kelly et al. 2014). The microbial communities are diverse and contribute to pathogen inhibition due to production of antimicrobial substances as well as competition for space and nutrients (Klaus et al. 2007; Rosenberg et al. 2007; Thurber et al. 2009). In our experiments, we found no distinct bacterial community changes after 4‐h salinity exposure, which contrasts the measured physiological reactions of coral host and algal symbiont. At the same time, Apprill et al. (2009) measured doubling times of 10+ hours for coral‐associated bacteria, which may have affected our ability to determine a bacterial microbiome response in the short‐term experiment. Conversely, we found no apparent physiological reaction, but pronounced microbial community changes after a 29‐day hypersalinity treatment. All corals, except those from the long‐term hypersalinity treatment, revealed highly uneven bacterial microbiomes that were numerically dominated by a single, distinct OTU (i.e. OTU0001) that could be identified to the level family, namely Rhodobacteraceae. Bacteria from this family were repeatedly observed in healthy corals (Sunagawa et al. 2009; Ceh et al. 2012; Morrow et al. 2012; Bayer et al. 2013; Kellogg et al. 2014; Li et al. 2014), even though they have also been found to be associated with stressed corals and stressed sea urchins (Buchan et al. 2005; Sunagawa et al. 2009; Meron et al. 2011a,b, 2012; Godwin et al. 2012; Pantos et al. 2015). Additionally, bacteria in the family Rhodobacteraceae have been found to be enriched in corals isolated from deeper habitats (27 m) compared to their shallow counterparts (6 m) (Pantos et al. 2015), which may explain their dominance in healthy F. granulosa collected in this study from a depth of 15–18 m. Taken together, the presence of Rhodobacteraceae in a range of hosts denotes environmental flexibility. For this reason, it is challenging to assign a specific role. However, the high abundance of a distinct OTU of this bacterial family in F. granulosa specimens from all treatments but the hypersalinity long‐term treatment indicates that this taxon probably provides an important function to the coral holobiont.
In contrast, Pseudomonas veronii, the ‘core’ microbiome member and the most abundant taxon in corals from the hypersalinity long‐term treatment, was present at a much lower abundance in corals from all other treatments (i.e. freshly collected coral, short‐term ambient salinity, short‐term hypersalinity and long‐term ambient salinity). As P. veronii was present in all corals, albeit at lower abundance, we argue that its increase under high salinity might signify a change of selection regime for this taxon under the altered environmental conditions and not an opportunistic association. The uniformity of all water samples, that is no significant differences in water samples over different treatments or time points, further supports a selective process for the changes in the coral microbiomes. The specific function of P. veronii remains to be determined. However, it seems to be a versatile taxon that has been isolated from distinct environments, for example natural freshwater springs, soil samples and wastewater filters where it has been shown to degrade a variety of simple aromatic organic compounds making it a beneficial bacterium for bioremediation of contaminated environments (Elomari et al. 1996; Nam et al. 2003; Onaca et al. 2007). More generally, bacteria in the genus Pseudomonas have repeatedly been shown to be abundant in hypersaline environments and display broad metabolic capacity (Fendrich 1988; Brusa et al. 2001; Sass et al. 2001; Isnansetyo & Kamei 2009).
Among other bacterial taxa that increased in abundance in the long‐term hypersalinity treatment, we identified the coral pathogen Vibrio shilonii (OTU0264) and also some unclassified Alteromonadaceae taxa (Table S3, Supporting information). These taxa are presumably associated with coral stress and disease, but are known to reside in healthy corals as well (Rosenberg & Falkovitz 2004; Sunagawa et al. 2009). Taken together, bacterial microbiome restructuring under highsalinity levels as signified by loss of the numerically dominant bacterial taxon (i.e. OTU0001), the increase in P. veronii (i.e. OTU0010), as well as an overall increase in richness, evenness and diversity possibly indicates stress (Bourne et al. 2008; Garren et al. 2009; Sunagawa et al. 2009; Meron et al. 2011a,b; Zhang et al. 2015). At the same time, major microbiome restructuring induced by environmental stress (i.e. high salinity) in the absence of a measurable physiological reaction of the coral holobiont may give support to the probiotic hypothesis (Reshef et al. 2006), that is a change of the microbiome to facilitate coral holobiont acclimation.
Functional changes of bacterial communities indicate metabolic adjustment
Mapping of differences in bacterial community composition to putative functional differences revealed a prominent increase in PHB storage as well as changes in nitrogen and sulphur cycling in long‐term hypersalinity samples in comparison with all other coral samples. PHB can be synthesized by microorganisms as a carbon reservoir in cells (Roberts 2005) and may be produced in response to various stressors, such as nutrient limitation, for example under nitrogen‐limiting conditions (Ayub et al. 2004; Soto et al. 2012). Interestingly, PHB has also been identified as an osmolyte in microorganisms (Doronina et al. 2000; Martin et al. 2002; Arora et al. 2006; Soto et al. 2012). Additionally, PHB production in Rhizobia with a potential benefit for plant cultivation in saline soil has been suggested (Arora et al. 2006; Ali et al. 2014). It is striking that Pseudomonas strains closely related to P. veronii are shown to produce PHB (Ayub et al. 2004; Yan et al. 2008; Soto et al. 2012), but even more so, the genome of P. veronii harbours the enzyme 3‐hydroxyisobutyrate dehydrogenase (Ramírez‐Bahena et al. 2015), which is part of the PHB metabolism (Hügler & Sievert 2011). This provides a putative functional link to the numerical dominance of P. veronii in the long‐term hypersalinity samples and potentially indicates functional adaptation/acclimation of the coral holobiont by alteration of its microbiome. Such functional changes were shown in the aphid Acyrthosiphon pisum where replacing the native gut bacteria Buchnera line LSR1 with line 5AY from a more thermotolerant aphid matriline conferred a dramatic increase in thermal tolerance (Moran & Yun 2015).
Changes in sulphur cycling as suggested by an upregulation of ‘sulphur oxidizer’ and a downregulation of ‘sulphate reducer’ presumably indicate the enrichment of oxidized products in the sulphur metabolism. The coral holobiont is a major contributor to the production of dimethylsulphide (DMS), a central compound of the global sulphur cycle (Raina et al. 2013), which can become oxidized to dimethylsulphoxide (DMSO) (Sunda et al. 2002). DMSO has a stronger reactivity towards reactive oxygen species (ROS) than DMS, is more hydrophilic (allowing higher cellular concentrations) and can be further oxidized to the water‐soluble antioxidant methane sulphinic acid (Sunda et al. 2002). Hence, an increased production (accompanied by a decreased reduction) of DMSO acting as an ROS scavenger may enable the coral to cope with increased oxidative stress in Symbiodinium. In agreement with these patterns, increased oxidative stress accompanied by antioxidant production as a response to high salinity has been shown for algae and other plants (Gossett et al. 1996; Fadzilla et al. 1997; Hernández et al. 2000; Jahnke & White 2003).
Another distinct pattern emerged from the metabolic profile of nitrogen‐related functions. We found processes that increase nitrogen availability for the holobiont to be enhanced (i.e. ‘dinitrogen‐fixing’ and ‘nitrogen fixation’), whereas processes that require the availability of fixed nitrogen were reduced (i.e. ‘ammonia oxidizer’ and ‘nitrite reducer’). This suggests an enhanced nutrient limitation of the coral holobiont (Rädecker et al. 2015). Nutrient limitation may be a consequence of an increased metabolism with enhanced nutrient requirements.
Long‐term coral holobiont response may indicate acclimation
Changes of the coral microbiome under changed environmental conditions were previously described (e.g. Klaus et al. 2007; Meron et al. 2012; Jessen et al. 2013; Kelly et al. 2014; Pantos et al. 2015), and that these changes are relevant to holobiont function was demonstrated by Moran & Yun (Moran & Yun 2015). In line with these studies, we interpret the here‐observed prevalent change of the coral microbiome in combination with a lack of an apparent stress response by the coral or symbiont in the long‐term hypersalinity treatment as indication for an acclimation response. This is supported by the putative functional changes we detected in the microbial community, that is upregulation of PHB as an osmolyte, alterations to the nitrogen cycle to compensate for nutrient deficiency, and synthesis of DMSO as a ROS scavenger. It is important to consider that the adjustments of the Fungia granulosa coral holobiont to a high‐salinity environment presumably require considerable energy and these energy requirements need to be taken into account when assessing the response of corals to changes in salinity. Taking the large biomass of the solitary coral F. granulosa into account, energy reserves may be sufficient for supposedly initial stress periods (as a response to the changing environmental conditions) and simultaneous acclimation. By comparison, commonly employed setups using small coral fragments in short‐term experiments may considerably underestimate coral resilience towards (salt) stress and might miss acclimation due to insufficient energy reserves of the coral fragment to sustain and acclimate to the stressor (Ferrier‐Pages et al. 1999; Kerswell & Jones 2003; Manzello & Lirman 2003; Chartrand et al. 2009; Lirman & Manzello 2009; Seveso et al. 2013).
Taken together, we argue that changes in salinities lead to changes in the holobionts internal environment, which in turn affect microbiome structure by selecting for a more advantageous bacterial community composition as posited by the coral probiotic hypothesis (Reshef et al. 2006). Future studies should target the temporal stability of restructured coral microbiomes accompanied by physiological measures under enduring ‘stress’ conditions to unequivocally confirm the importance of the microbiome to coral holobiont function.
Conflict of interest
The authors declared that they have no conflict of interest.
T.R., C.R.V. and R.v.d.M. designed and conceived the experiments. T.R. and M.A.O. generated the data. T.R., C.R.V., M.A.O. and A.R. analysed and interpreted the data. C.R.V. and R.v.d.M. contributed reagents/materials/analysis tools. T.R. and C.R.V. wrote the manuscript.
Data accessibility
Sequences determined in this study have been deposited in the NCBI Sequence Read Archive under accession no. PRJNA282461 (http://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA282461).
Supporting information
Fig. S1 Seawater reverse osmosis (SWRO) desalination plant discharge structure and 25 m transect.
Fig. S2 Increased mucus production and bubble formation of the coral Fungia granulosa after 4 h incubation at (a) 39 PSU (b) 55 PSU.
Fig. S3 Clustering of water samples based on Bray–Curtis dissimilarity of microbial community abundances in a principal coordinate analysis (PCoA) (R 2 = 0.89).
Fig. S4 Relative absence/presence of microbial genes associated with (A, B) PHB metabolism and (C, D) sulphur cycling via picrust.
Table S1 Correlation between coral microbiomes and environmental parameters.
Table S2 OTU abundance counts over samples with annotation and reference OTU sequence.
Table S3 OTUs enriched in fresh, short‐term ambient and hypersaline, and long‐term ambient corals (P ≤ 0.001, average abundance ≥10); OTUs enriched in long‐term hypersaline corals (P ≤ 0.001, average abundance ≥10; abundance count in each of the three replicates).
Acknowledgements
We would like to thank CMOR for assistance and support in field operations. We thank the KAUST Marine Genomics class 2014 (Ghaida Hadaidi, Guoxin Cui, Manalle Al‐Salamah, Manuel Aranda, Marcela Herrera, Noura Zahran) for their assistance in field and laboratory work. We thank Craig Michell for support in sequencing preparations, Nils Rädecker for help in interpreting holobiont nitrogen cycling, and Shobhit Agrawal and Maren Ziegler for assistance with the bacterial function analysis. This work was supported by King Abdullah University of Science and Technology (KAUST), Center Competitive Funding (CCF) Program URF/1/1973‐02.
References
- Ali AA, Shaban KA, Tantawy EA (2014) Effect of poly‐β‐hydroxybutyrate (PHB) and glycogen producing endophytic bacteria on yield, growth and nutrient. Applied Science Reports, 8, 134–142. [Google Scholar]
- Alutoin S, Boberg J, Nyström M, Tedengren M (2001) Effects of the multiple stressors copper and reduced salinity on the metabolism of the hermatypic coral Porites lutea . Marine Environment Research, 52, 289–299. [DOI] [PubMed] [Google Scholar]
- Andersson AF, Lindberg M, Jakobsson H et al (2008) Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One, 3, e2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apprill A, Marlow HQ, Martindale MQ, Rappe MS (2009) The onset of microbial associations in the coral Pocillopora meandrina . ISME Journal, 3, 685–699. [DOI] [PubMed] [Google Scholar]
- Arndt D, Xia J, Liu Y et al (2012) METAGENassist: a comprehensive web server for comparative metagenomics. Nucleic Acids Research, 40, W88–W95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora NK, Singhal V, Maheshwari DK (2006) Salinity‐induced accumulation of poly‐β‐hydroxybutyrate in rhizobia indicating its role in cell protection. World Journal of Microbiology and Biotechnology, 22, 603–606. [Google Scholar]
- Ayub ND, Pettinari MJ, Ruiz JA, López NI (2004) A polyhydroxybutyrate‐producing Pseudomonas sp. isolated from antarctic environments with high stress resistance. Current Microbiology, 49, 170–174. [DOI] [PubMed] [Google Scholar]
- Barott KL, Rodriguez‐Brito B, Janouškovec J et al (2011) Microbial diversity associated with four functional groups of benthic reef algae and the reef‐building coral Montastraea annularis . Environmental Microbiology, 13, 1192–1204. [DOI] [PubMed] [Google Scholar]
- Bayer T, Neave MJ, Alsheikh‐Hussain A et al (2013) The microbiome of the Red Sea coral Stylophora pistillata is dominated by tissue‐associated Endozoicomonas bacteria. Applied and Environmental Microbiology, 79, 4759–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne D, Webster N (2013) Coral reef bacterial communities In: The Prokaryotes (eds Rosenberg E, DeLong E, Lory S, Stackebrandt E, Thompson F.), pp. 163–187. Springer, Berlin and Heidelberg. [Google Scholar]
- Bourne D, Iida Y, Uthicke S, Smith‐Keune C (2008) Changes in coral‐associated microbial communities during a bleaching event. ISME Journal, 2, 350–363. [DOI] [PubMed] [Google Scholar]
- Bouvier TC, del Giorgio PA (2002) Compositional changes in free‐living bacterial communities along a salinity gradient in two temperate estuaries. Limnology and Oceanography, 47, 453–470. [Google Scholar]
- Brusa T, Borin S, Ferrari F et al (2001) Aromatic hydrocarbon degradation patterns and catechol 2,3‐dioxygenase genes in microbial cultures from deep anoxic hypersaline lakes in the eastern Mediterranean sea. Microbiological Research, 156, 49–58. [DOI] [PubMed] [Google Scholar]
- Buchan A, González JM, Moran MA (2005) Overview of the marine roseobacter lineage. Applied and Environment Microbiology, 71, 5665–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres MD, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology, 90, 3566–3574. [DOI] [PubMed] [Google Scholar]
- Ceh J, Raina J‐B, Soo RM, van Keulen M, Bourne DG (2012) Coral‐bacterial communities before and after a coral mass spawning event on Ningaloo Reef. PLoS One, 7, e36920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick‐Furman NE, Goffredo S, Loya Y (2000) Growth and population dynamic model of the reef coral Fungia granulosa Klunzinger, 1879 at Eilat, northern Red Sea. Journal of Experimental Marine Biology and Ecology, 249, 199–218. [DOI] [PubMed] [Google Scholar]
- Chao A (1984) Nonparametric estimation of the number of classes in a population. Scandinavian Journal of Statistics, 11, 265–270. [Google Scholar]
- Chartrand K, Durako M, Blum J (2009) Effect of hyposalinity on the photophysiology of Siderastrea radians . Marine Biology, 156, 1691–1702. [Google Scholar]
- Clarke K, Gorley R (2006) PRIMER V6: User Manual/Tutorial. Primer‐E Ltd, Ivybridge, UK. [Google Scholar]
- Coles SL (2003) Coral species diversity and environmental factors in the Arabian Gulf and the Gulf of Oman: a comparison to the Indo‐Pacific region. Atoll Research Bulletin, 507, 1–13. [Google Scholar]
- Csonka LN, Hanson AD (1991) Prokaryotic osmoregulation: genetics and physiology. Annual Review of Microbiology, 45, 569–606. [DOI] [PubMed] [Google Scholar]
- Doronina NV, Trotsenko YA, Tourova TP (2000) Methylarcula marina gen. nov., sp. nov. and Methylarcula terricola sp. nov.: novel aerobic, moderately halophilic, facultatively methylotrophic bacteria from coastal saline environments. International Journal of Systematic and Evolutionary Microbiology, 50, 1849–1859. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 27, 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge SE, Shearer TL, Morgan MB, Snell TW (2013) Sub‐lethal coral stress: detecting molecular responses of coral populations to environmental conditions over space and time. Aquatic Toxicology, 128–129, 135–146. [DOI] [PubMed] [Google Scholar]
- Elomari M, Coroler L, Hoste B et al (1996) DNA relatedness among Pseudomonas strains isolated from natural mineral waters and proposal of Pseudomonas veronii sp. nov. International Journal of Systematic Bacteriology, 46, 1138–1144. [DOI] [PubMed] [Google Scholar]
- Evans DH (2008) Osmotic and Ionic Regulation: Cells and Animals. CRC Press, Boca Raton, Florida. [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics, 131, 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadzilla NAM, Finch RP, Burdon RH (1997) Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. Journal of Experimental Botany, 48, 325–331. [Google Scholar]
- Fendrich C (1988) Halovibrio variabilis gen. nov. sp. nov., Pseudomonas halophila sp. nov. and a new halophilic aerobic coccoid Eubacterium from Great Salt Lake, Utah, USA. Systematic and Applied Microbiology, 11, 36–43. [Google Scholar]
- Ferrier‐Pages C, Gattuso J‐P, Jaubert J (1999) Effect of small variations in salinity on the rates of photosynthesis and respiration of the zooxanthellate coral Stylophora pistillata . Marine Ecology Progress Series, 181, 309–314. [Google Scholar]
- Garren M, Raymundo L, Guest J, Harvell CD, Azam F (2009) Resilience of coral‐associated bacterial communities exposed to fish farm effluent. PLoS One, 4, e7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattuso J‐P, Allemand D, Frankignoulle M (1999) Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. American Zoologist, 39, 160–183. [Google Scholar]
- Genty B, Briantais J‐M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA)‐General Subjects, 990, 87–92. [Google Scholar]
- Godwin S, Bent E, Borneman J, Pereg L (2012) The role of coral‐associated bacterial communities in Australian subtropical white syndrome of Turbinaria mesenterina . PLoS One, 7, e44243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika, 40, 237–264. [Google Scholar]
- Gossett DR, Banks SW, Millhollon EP, Lucas MC (1996) Antioxidant response to NaCl stress in a control and an NaCl‐tolerant cotton cell line grown in the presence of paraquat, buthionine sulfoximine, and exogenous glutathione. Plant Physiology, 112, 803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasshoff K, Kremling K, Ehrhardt M (2009) Methods of Seawater Analysis. John Wiley & Sons, Hoboken, New Jersey. [Google Scholar]
- Hackstadt AJ, Hess AM (2009) Filtering for increased power for microarray data analysis. BMC Bioinformatics, 10, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hédouin L, Pilon R, Puisay A (2015) Hyposalinity stress compromises the fertilization of gametes more than the survival of coral larvae. Marine Environment Research, 104, 1–9. [DOI] [PubMed] [Google Scholar]
- Hernández JA, Jiménez A, Mullineaux P, Sevilia F (2000) Tolerance of pea (Pisum sativum L.) to long‐term salt stress is associated with induction of antioxidant defences. Plant, Cell and Environment, 23, 853–862. [Google Scholar]
- Hoegh‐Guldberg O, Smith GJ (1989) The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata Esper and Seriatopora hystrix Dana. Journal of Experimental Marine Biology and Ecology, 129, 279–303. [Google Scholar]
- Hügler M, Sievert SM (2011) Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Marine Science, 3, 261–289. [DOI] [PubMed] [Google Scholar]
- Huse SM, Welch DM, Morrison HG, Sogin ML (2010) Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environmental Microbiology, 12, 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnansetyo A, Kamei Y (2009) Bioactive substances produced by marine isolates of Pseudomonas . Journal of Industrial Microbiology & Biotechnology, 36, 1239–1248. [DOI] [PubMed] [Google Scholar]
- Jahnke LS, White AL (2003) Long‐term hyposaline and hypersaline stresses produce distinct antioxidant responses in the marine alga Dunaliella tertiolecta . Journal of Plant Physiology, 160, 1193–1202. [DOI] [PubMed] [Google Scholar]
- Jessen C, Villa Lizcano JF, Bayer T et al (2013) In‐situ effects of eutrophication and overfishing on physiology and bacterial diversity of the Red Sea Coral Acropora hemprichii . PLoS One, 8, e62091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg CA, Piceno YM, Tom LM et al (2014) Comparing bacterial community composition of HEALTHY AND DARK SPOT‐AFFECTED Siderastrea siderea in Florida and the Caribbean. PLoS One, 9, e108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly LW, Williams GJ, Barott KL et al (2014) Local genomic adaptation of coral reef‐associated microbiomes to gradients of natural variability and anthropogenic stressors. Proceedings of the National Academy of Sciences of the United States of America, 111, 10227–10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerswell AP, Jones RJ (2003) Effects of hypo‐osmosis on the coral Stylophora pistillata: nature and cause of ‘low‐salinity bleaching’. Marine Ecology Progress Series, 253, 145–154. [Google Scholar]
- Klaus JS, Janse I, Heikoop JM, Sanford RA, Fouke BW (2007) Coral microbial communities, zooxanthellae and mucus along gradients of seawater depth and coastal pollution. Environmental Microbiology, 9, 1291–1305. [DOI] [PubMed] [Google Scholar]
- Langille MGI, Zaneveld J, Caporaso JG et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature Biotechnology, 31, 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema KA, Willis BL, Bourne DG (2012) Corals form characteristic associations with symbiotic nitrogen‐fixing bacteria. Applied and Environmental Microbiology, 78, 3136–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser MP, Mazel CH, Gorbunov MY, Falkowski PG (2004) Discovery of symbiotic nitrogen‐fixing Cyanobacteria in corals. Science, 305, 997–1000. [DOI] [PubMed] [Google Scholar]
- Li J, Chen Q, Long L‐J et al (2014) Bacterial dynamics within the mucus, tissue and skeleton of the coral Porites lutea during different seasons. Scientific Reports, 4, 7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirman D, Manzello D (2009) Patterns of resistance and resilience of the stress‐tolerant coral Siderastrea radians (Pallas) to sub‐optimal salinity and sediment burial. Journal of Experimental Marine Biology and Ecology, 369, 72–77. [Google Scholar]
- Manzello D, Lirman D (2003) The photosynthetic resilience of Porites furcata to salinity disturbance. Coral Reefs, 22, 537–540. [Google Scholar]
- Martin D, Bartlett D, Roberts M (2002) Solute accumulation in the deep‐sea bacterium Photobacterium profundum . Extremophiles, 6, 507–514. [DOI] [PubMed] [Google Scholar]
- Mayfield AB, Gates RD (2007) Osmoregulation in anthozoan–dinoflagellate symbiosis. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 147, 1–10. [DOI] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J et al (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME Journal, 6, 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meron D, Atias E, Iasur Kruh L et al (2011a) The impact of reduced pH on the microbial community of the coral Acropora eurystoma . ISME Journal, 5, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meron D, Hazanov L, Fine M, Banin E (2011b) Effect of ocean acidification on the coral microbial community In: Beneficial Microorganisms in Multicellular Life Forms (eds Rosenberg E, Gophna U.), pp. 163–173. Springer, Berlin and Heidelberg. [Google Scholar]
- Meron D, Rodolfo‐Metalpa R, Cunning R et al (2012) Changes in coral microbial communities in response to a natural pH gradient. ISME Journal, 6, 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe R, Bleninger T, Acevedo‐Feliz D, Lattemann S, Amy G (2014a) Combining autonomous underwater vehicle missions with velocity and salinity measurements for the evaluation of a submerged offshore SWRO concentrate discharge. Journal of Applied Water Engineering and Research, 2, 118–139. [Google Scholar]
- van der Merwe R, Röthig T, Voolstra CR et al (2014b) High salinity tolerance of the Red Sea coral Fungia granulosa under desalination concentrate discharge conditions: an in situ photophysiology experiment. Frontiers in Marine Science, 58, 1–8. [Google Scholar]
- Moberg F, Nyström M, Kautsky N, Tedengren M, Jarayabhand P (1997) Effects of reduced salinity on the rates of photosynthesis and respiration in the hermatypic corals Porites lutea and Pocillopora damicornis . Marine Ecology Progress Series, 157, 53–59. [Google Scholar]
- Moran NA, Yun Y (2015) Experimental replacement of an obligate insect symbiont. Proceedings of the National Academy of Sciences of the United States of America, 112, 2093–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow KM, Moss AG, Chadwick NE, Liles MR (2012) Bacterial associates of two Caribbean coral species reveal species‐specific distribution and geographic variability. Applied and Environmental Microbiology, 78, 6438–6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthiga NA, Szmant AM (1987) The effects of salinity stress on the rates of aerobic respiration and photosynthesis in the hermatypic coral Siderastrea siderea . Biological Bulletin, 173, 539–551. [DOI] [PubMed] [Google Scholar]
- Nam IH, Chang YS, Hong HB, Lee YE (2003) A novel catabolic activity of Pseudomonas veronii in biotransformation of pentachlorophenol. Applied Microbiology and Biotechnology, 62, 284–290. [DOI] [PubMed] [Google Scholar]
- Onaca C, Kieninger M, Engesser K‐H, Altenbuchner J (2007) Degradation of alkyl methyl ketones by Pseudomonas veronii MEK700. Journal of Bacteriology, 189, 3759–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantos O, Bongaerts P, Dennis PG, Tyson GW, Hoegh‐Guldberg O (2015) Habitat‐specific environmental conditions primarily control the microbiomes of the coral Seriatopora hystrix . ISME Journal, 9, 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruesse E, Quast C, Knittel K et al (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Research, 35, 7188–7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2012. ISBN 3‐900051‐07‐0. [Google Scholar]
- Rädecker N, Pogoreutz C, Voolstra CR, Wiedenmann J, Wild C (2015) Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends in Microbiology, 23, 490–497. [DOI] [PubMed] [Google Scholar]
- Raina J‐B, Tapiolas D, Willis BL, Bourne DG (2009) Coral‐associated bacteria and their role in the biogeochemical cycling of sulfur. Applied and Environmental Microbiology, 75, 3492–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina J‐B, Tapiolas DM, Foret S et al (2013) DMSP biosynthesis by an animal and its role in coral thermal stress response. Nature, 502, 677–680. [DOI] [PubMed] [Google Scholar]
- Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquatic Botany, 82, 222–237. [Google Scholar]
- Ramírez‐Bahena M‐H, Cuesta MJ, Tejedor C et al (2015) Pseudomonas endophytica sp. nov., isolated from stem tissue of Solanum tuberosum L. in Spain. International Journal of Systematic and Evolutionary Microbiology, 65, 2110–2117. [DOI] [PubMed] [Google Scholar]
- Reaka‐Kudla ML, Wilson DE, Wilson EO (1996) Biodiversity II: Understanding and Protecting Our Biological Resources. Joseph Henry Press, Washington, District of Columbia. [Google Scholar]
- Reshef L, Koren O, Loya Y, Zilber‐Rosenberg I, Rosenberg E (2006) The coral probiotic hypothesis. Environmental Microbiology, 8, 2068–2073. [DOI] [PubMed] [Google Scholar]
- Ritchie K (2011) Bacterial symbionts of corals and Symbiodinium In: Beneficial Microorganisms in Multicellular Life Forms (eds Rosenberg E, Gophna U.), pp. 139–150. Springer‐Verlag, Berlin and Heidelberg. [Google Scholar]
- Roberts MF (2005) Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Systems, 1, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DA, Johnston EL, Knott NA (2010) Impacts of desalination plant discharges on the marine environment: a critical review of published studies. Water Research, 44, 5117–5128. [DOI] [PubMed] [Google Scholar]
- Rohwer F, Seguritan V, Azam F, Knowlton N (2002) Diversity and distribution of coral‐associated bacteria. Marine Ecology Progress Series, 243, 1–10. [Google Scholar]
- Rosenberg E, Falkovitz L (2004) The Vibrio shiloi/Oculina patagonica model system of coral bleaching. Annual Review of Microbiology, 58, 143–159. [DOI] [PubMed] [Google Scholar]
- Rosenberg E, Koren O, Reshef L, Efrony R, Zilber‐Rosenberg I (2007) The role of microorganisms in coral health, disease and evolution. Nature Reviews Microbiology, 5, 355–362. [DOI] [PubMed] [Google Scholar]
- Sass AM, Sass H, Coolen MJL, Cypionka H, Overmann J (2001) Microbial communities in the chemocline of a hypersaline deep‐sea basin (Urania Basin, Mediterranean Sea). Applied and Environmental Microbiology, 67, 5392–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawall Y, Teichberg MC, Seemann J et al (2011) Nutritional status and metabolism of the coral Stylophora subseriata along a eutrophication gradient in Spermonde Archipelago (Indonesia). Coral Reefs, 30, 841–853. [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open‐source, platform‐independent, community‐supported software for describing and comparing microbial communities. Applied and Environmental Microbiology, 75, 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K, Erez J (2006) The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma . Limnology and Oceanography, 51, 1284–1293. [Google Scholar]
- Seveso D, Montano S, Strona G et al (2013) Exploring the effect of salinity changes on the levels of Hsp60 in the tropical coral Seriatopora caliendrum . Marine Environment Research, 90, 96–103. [DOI] [PubMed] [Google Scholar]
- Shick MJ (1991) A Functional Biology of Sea Anemones. Chapman & Hall, UK. [Google Scholar]
- Soto G, Setten L, Lisi C et al (2012) Hydroxybutyrate prevents protein aggregation in the halotolerant bacterium Pseudomonas sp. CT13 under abiotic stress. Extremophiles, 16, 455–462. [DOI] [PubMed] [Google Scholar]
- Sunagawa S, DeSantis TZ, Piceno YM et al (2009) Bacterial diversity and White Plague Disease‐associated community changes in the Caribbean coral Montastraea faveolata. ISME Journal, 3, 512–521. [DOI] [PubMed] [Google Scholar]
- Sunda W, Kieber DJ, Kiene RP, Huntsman S (2002) An antioxidant function for DMSP and DMS in marine algae. Nature, 418, 317–320. [DOI] [PubMed] [Google Scholar]
- Thurber RV, Willner‐Hall D, Rodriguez‐Mueller B et al (2009) Metagenomic analysis of stressed coral holobionts. Environmental Microbiology, 11, 2148–2163. [DOI] [PubMed] [Google Scholar]
- True JD (2012) Salinity as a structuring force for near shore coral communities In: Proceedings of the 12th International Coral Reef Symposium (eds Yellowlees D, Hughes T.), pp. 9–13. Cairns, Queensland. [Google Scholar]
- Wilkinson C (2008) Status of Coral Reefs of the World: 2008. Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre, Townsville, Queensland. [Google Scholar]
- Yan Y, Yang J, Dou Y et al (2008) Nitrogen fixation island and rhizosphere competence traits in the genome of root‐associated Pseudomonas stutzeri A1501. Proceedings of the National Academy of Sciences of the United States of America, 105, 7564–7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey PH, Blake WR, Conley J (2002) Unusual organic osmolytes in deep‐sea animals: adaptations to hydrostatic pressure and other perturbants. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 133, 667–676. [DOI] [PubMed] [Google Scholar]
- Zhang Y‐Y, Ling J, Yang Q‐S et al (2015) The diversity of coral associated bacteria and the environmental factors affect their community variation. Ecotoxicology, 24, 1467–1477 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Seawater reverse osmosis (SWRO) desalination plant discharge structure and 25 m transect.
Fig. S2 Increased mucus production and bubble formation of the coral Fungia granulosa after 4 h incubation at (a) 39 PSU (b) 55 PSU.
Fig. S3 Clustering of water samples based on Bray–Curtis dissimilarity of microbial community abundances in a principal coordinate analysis (PCoA) (R 2 = 0.89).
Fig. S4 Relative absence/presence of microbial genes associated with (A, B) PHB metabolism and (C, D) sulphur cycling via picrust.
Table S1 Correlation between coral microbiomes and environmental parameters.
Table S2 OTU abundance counts over samples with annotation and reference OTU sequence.
Table S3 OTUs enriched in fresh, short‐term ambient and hypersaline, and long‐term ambient corals (P ≤ 0.001, average abundance ≥10); OTUs enriched in long‐term hypersaline corals (P ≤ 0.001, average abundance ≥10; abundance count in each of the three replicates).
Data Availability Statement
Sequences determined in this study have been deposited in the NCBI Sequence Read Archive under accession no. PRJNA282461 (http://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA282461).


